Page 30 - Read Online
P. 30
Felton et al. J Cancer Metastasis Treat 2018;4:51 I http://dx.doi.org/10.20517/2394-4722.2018.39 Page 7 of 9
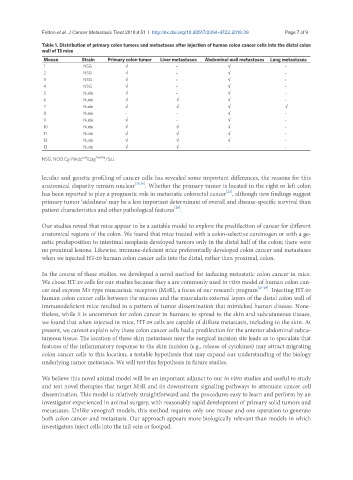
Table 1. Distribution of primary colon tumors and metastases after injection of human colon cancer cells into the distal colon
wall of 13 mice
Mouse Strain Primary colon tumor Liver metastases Abdominal wall metastases Lung metastases
1 NSG √ - √ -
2 NSG √ - √ -
3 NSG √ - √ -
4 NSG √ - √ -
5 Nude √ - √ -
6 Nude √ √ √ -
7 Nude √ √ √ √
8 Nude - - √ -
9 Nude √ - √ -
10 Nude √ √ √ -
11 Nude √ √ √ -
12 Nude √ √ √ -
13 Nude √ √ - -
scid
NSG: NOD.Cg-Prkdc Il2rg Tim1Wji /SzJ.
lecular and genetic profiling of cancer cells has revealed some important differences, the reasons for this
anatomical disparity remain unclear [25,26] . Whether the primary tumor is located in the right or left colon
[25]
has been reported to play a prognostic role in metastatic colorectal cancer , although new findings suggest
primary tumor ‘sidedness’ may be a less important determinant of overall and disease-specific survival than
[26]
patient characteristics and other pathological features .
Our studies reveal that mice appear to be a suitable model to explore the predilection of cancer for different
anatomical regions of the colon. We found that mice treated with a colon-selective carcinogen or with a ge-
netic predisposition to intestinal neoplasia developed tumors only in the distal half of the colon; there were
no proximal lesions. Likewise, immune-deficient mice preferentially developed colon cancer and metastases
when we injected HT-29 human colon cancer cells into the distal, rather than proximal, colon.
In the course of these studies, we developed a novel method for inducing metastatic colon cancer in mice.
We chose HT-29 cells for our studies because they a are commonly used in vitro model of human colon can-
cer and express M3 type muscarinic receptors (M3R), a focus of our research program [27-29] . Injecting HT-29
human colon cancer cells between the mucosa and the muscularis external layers of the distal colon wall of
immunodeficient mice resulted in a pattern of tumor dissemination that mimicked human disease. None-
theless, while it is uncommon for colon cancer in humans to spread to the skin and subcutaneous tissues,
we found that when injected in mice, HT-29 cells are capable of diffuse metastasis, including to the skin. At
present, we cannot explain why these colon cancer cells had a predilection for the anterior abdominal subcu-
taneous tissue. The location of these skin metastases near the surgical incision site leads us to speculate that
features of the inflammatory response to the skin incision (e.g., release of cytokines) may attract migrating
colon cancer cells to this location, a testable hypothesis that may expand our understanding of the biology
underlying tumor metastasis. We will test this hypothesis in future studies.
We believe this novel animal model will be an important adjunct to our in vitro studies and useful to study
and test novel therapies that target M3R and its downstream signaling pathways to attenuate cancer cell
dissemination. This model is relatively straightforward and the procedures easy to learn and perform by an
investigator experienced in animal surgery, with reasonably rapid development of primary solid tumors and
metastases. Unlike xenograft models, this method requires only one mouse and one operation to generate
both colon cancer and metastasis. Our approach appears more biologically relevant than models in which
investigators inject cells into the tail vein or footpad.