Page 702 - Read Online
P. 702
Merle et al. Hepatoma Res 2020;6:60 I http://dx.doi.org/10.20517/2394-5079.2020.52 Page 7 of 10
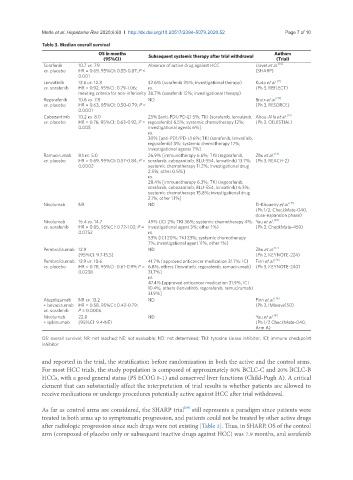
Table 3. Median overall survival
OS in months Authors
(95%CI) Subsequent systemic therapy after trial withdrawal (Trial)
Sorafenib 10.7 vs. 7.9 Absence of active drug against HCC Llovet et al. [10]
vs. placebo HR = 0.69, 95%CI: 0.55-0.87; P < (SHARP)
0.001
Lenvatinib 13.6 vs. 12.3 32.6% (sorafenib 25%; investigational therapy) Kudo et al. [11]
vs. sorafenib HR = 0.92, 95%CI: 0.79-1.06; vs. (Ph 3, REFLECT)
meeting criteria for non-inferiority 38.7% (sorafenib 12%; investigational therapy)
Regorafenib 10.6 vs. 7.8 ND Bruix et al. [12]
vs. placebo HR = 0.63, 95%CI: 0.50-0.79; P < (Ph 3, RESORCE)
0.0001
Cabozantinib 10.2 vs. 8.0 25% [anti-PD1/PD-L1 5%; TKI (sorafenib, lenvatinib, Abou-Alfa et al. [13]
vs. placebo HR = 0.76, 95%CI: 0.63-0.92; P = regorafenib) 6.5%; systemic chemotherapy 12%; (Ph 3, CELESTIAL)
0.005 investigational agents 6%]
vs.
30% [anti-PD1/PD-L1 6%; TKI (sorafenib, lenvatinib,
regorafenib) 3%; systemic chemotherapy 17%;
investigational agents 7%]
Ramucirumab 8.1 vs. 5.0 26.9% [immunotherapy 6.6%; TKI (regorafenib, Zhu et al. [14]
vs. placebo HR = 0.69, 95%CI: 0.57-0.84; P = sorafenib, cabozantinib, BLU-554, lenvatinib) 13.7%; (Ph 3, REACH-2)
0.0002 systemic chemotherapy 11.2%; investigational drug
2.5%; other 0.5%]
vs.
28.4% [immunotherapy 6.3%; TKI (regorafenib,
sorafenib, cabozantinib, BLU-554, lenvatinib) 6.3%;
systemic chemotherapy 15.8%; investigational drug
2.1%; other 1.1%]
Nivolumab NR ND El-Khoueiry et al. [19]
(Ph 1/2, CheckMate-040,
dose-expansion phase)
Nivolumab 16.4 vs. 14.7 49% (ICI 2%; TKI 36%; systemic chemotherapy 4%; Yau et al. [20]
vs. sorafenib HR = 0.85, 95%CI: 0.72-1.02; P = investigational agent 3%; other 1%) (Ph 3, CheckMate-459)
0.0752 vs.
53% (ICI 20%; TKI 23%; systemic chemotherapy
7%; investigational agent 11%; other 1%)
Pembrolizumab 12.9 ND Zhu et al. [17]
(95%CI: 9.7-15.5) (Ph 2, KEYNOTE-224)
Pembrolizumab 13.9 vs. 10.6 41.7% [approved anticancer medication 31.7%: ICI Finn et al. [18]
vs. placebo HR = 0.78, 95%CI: 0.61-0.99; P = 6.8%, others (lenvatinib, regorafenib, ramucirumab) (Ph 3, KEYNOTE-240)
0.0238 31.7%]
vs.
47.4% [approved anticancer medication 31.9%, ICI
10.4%, others (lenvatinib, regorafenib, ramucirumab)
31.9%]
Atezolizumab NR vs. 13.2 ND Finn et al. [16]
+ bevacizumab HR = 0.58, 95%CI: 0.42-0.79; (Ph 3, IMbrave150)
vs. sorafenib P = 0.0006
Nivolumab 22.8 ND Yau et al. [8]
+ ipilimumab (95%CI: 9.4-NE) (Ph 1/2 CheckMate-040,
Arm A)
OS: overall survival; NR: not reached; NE: not evaluable; ND: not determined; TKI: tyrosine kinase inhibitor; ICI: immune checkpoint
inhibitor
and reported in the trial, the stratification before randomization in both the active and the control arms.
For most HCC trials, the study population is composed of approximately 80% BCLC-C and 20% BCLC-B
HCCs, with a good general status (PS ECOG 0-1) and conserved liver functions (Child-Pugh A). A critical
element that can substantially affect the interpretation of trial results is whether patients are allowed to
receive medications or undergo procedures potentially active against HCC after trial withdrawal.
[10]
As far as control arms are considered, the SHARP trial still represents a paradigm since patients were
treated in both arms up to symptomatic progression, and patients could not be treated by other active drugs
after radiologic progression since such drugs were not existing [Table 3]. Thus, in SHARP, OS of the control
arm (composed of placebo only or subsequent inactive drugs against HCC) was 7.9 months, and sorafenib