Page 622 - Read Online
P. 622
Ichida et al. Hepatoma Res 2020;6:54 I http://dx.doi.org/10.20517/2394-5079.2020.59 Page 7 of 11
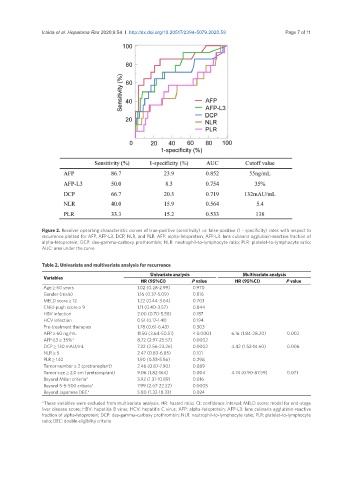
Figure 2. Receiver operating characteristic curves of true-positive (sensitivity) vs. false-positive (1 - specificity) rates with respect to
recurrence plotted for AFP, AFP-L3, DCP, NLR, and PLR. AFP: alpha-fetoprotein; AFP-L3: lens culinaris agglutinin-reactive fraction of
alpha-fetoprotein; DCP: des-gamma-carboxy prothrombin; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio;
AUC: area under the curve
Table 2. Univariate and multivariate analysis for recurrence
Univariate analysis Multivariate analysis
Variables
HR (95%CI) P value HR (95%CI) P value
Age ≥ 60 years 1.02 (0.28-2.99) 0.970
Gender (male) 1.16 (0.37-5.09) 0.816
MELD score ≥ 12 1.22 (0.44-3.64) 0.703
Child-pugh score ≥ 9 1.11 (0.40-3.57) 0.844
HBV infection 2.00 (0.70-5.58) 0.187
HCV infection 0.51 (0.17-1.41) 0.194
Pre-treatment therapies 1.78 (0.61-6.43) 0.303
AFP ≥ 60 ng/mL 11.50 (3.64-50.51) < 0.0001 6.16 (1.84-28.20) 0.002
AFP-L3 ≥ 35%* 8.72 (2.97-25.57) 0.0002
DCP ≥ 130 mAU/mL 7.22 (2.56-23.26) 0.0002 4.42 (1.52-14.60) 0.006
NLR ≥ 5 2.47 (0.83-6.85) 0.101
PLR ≥ 140 1.90 (0.53-5.56) 0.298
Tumor number ≥ 2 (pretransplant) 2.46 (0.87-7.90) 0.089
Tumor size ≥ 2.0 cm (pretransplant) 9.06 (1.82-164) 0.004 4.74 (0.90-87.59) 0.071
Beyond Milan criteria* 3.92 (1.31-10.89) 0.016
Beyond 5-5-500 criteria* 7.99 (2.67-22.22) 0.0005
Beyond Japanese DEC* 5.80 (1.32-18.33) 0.024
*These variables were excluded from multivariate analysis. HR: hazard ratio; CI: confidence interval; MELD score: model for end-stage
liver disease score; HBV: hepatitis B virus; HCV: hepatitis C virus; AFP: alpha-fetoprotein; AFP-L3: lens culinaris agglutinin-reactive
fraction of alpha-fetoprotein; DCP: des-gamma-carboxy prothrombin; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte
ratio; DEC: double eligibility criteria