Page 546 - Read Online
P. 546
Boortalary et al. Hepatoma Res 2020;6:48 I http://dx.doi.org/10.20517/2394-5079.2020.38 Page 3 of 7
A B
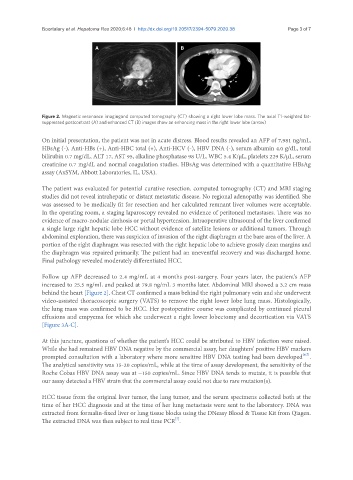
Figure 2. Magnetic resonance imagingand computed tomography (CT) showing a right lower lobe mass. The axial T1-weighted fat-
suppressed postcontrast (A) and enhanced CT (B) images show an enhancing mass in the right lower lobe (arrow)
On initial presentation, the patient was not in acute distress. Blood results revealed an AFP of 7,981 ng/mL,
HBsAg (-), Anti-HBs (+), Anti-HBC total (+), Anti-HCV (-), HBV DNA (-), serum albumin 4.0 g/dL, total
bilirubin 0.7 mg/dL, ALT 17, AST 95, alkaline phosphatase 98 U/L, WBC 5.4 K/µL, platelets 229 K/µL, serum
creatinine 0.7 mg/dL and normal coagulation studies. HBsAg was determined with a quantitative HBsAg
assay (AxSYM, Abbott Laboratories, IL, USA).
The patient was evaluated for potential curative resection. computed tomography (CT) and MRI staging
studies did not reveal intrahepatic or distant metastatic disease. No regional adenopathy was identified. She
was assessed to be medically fit for resection and her calculated remnant liver volumes were acceptable.
In the operating room, a staging laparoscopy revealed no evidence of peritoneal metastases. There was no
evidence of macro-nodular cirrhosis or portal hypertension. Intraoperative ultrasound of the liver confirmed
a single large right hepatic lobe HCC without evidence of satellite lesions or additional tumors. Through
abdominal exploration, there was suspicion of invasion of the right diaphragm at the bare area of the liver. A
portion of the right diaphragm was resected with the right hepatic lobe to achieve grossly clean margins and
the diaphragm was repaired primarily. The patient had an uneventful recovery and was discharged home.
Final pathology revealed moderately differentiated HCC.
Follow up AFP decreased to 2.4 mg/mL at 4 months post-surgery. Four years later, the patient’s AFP
increased to 25.5 ng/mL and peaked at 79.8 ng/mL 3 months later. Abdominal MRI showed a 3.2 cm mass
behind the heart [Figure 2]. Chest CT confirmed a mass behind the right pulmonary vein and she underwent
video-assisted thoracoscopic surgery (VATS) to remove the right lower lobe lung mass. Histologically,
the lung mass was confirmed to be HCC. Her postoperative course was complicated by continued pleural
effusions and empyema for which she underwent a right lower lobectomy and decortication via VATS
[Figure 3A-C].
At this juncture, questions of whether the patient’s HCC could be attributed to HBV infection were raised.
While she had remained HBV DNA negative by the commercial assay, her daughters’ positive HBV markers
[6,7]
prompted consultation with a laboratory where more sensitive HBV DNA testing had been developed .
The analytical sensitivity was 15-20 copies/mL, while at the time of assay development, the sensitivity of the
Roche Cobas HBV DNA assay was at ~150 copies/mL. Since HBV DNA tends to mutate, it is possible that
our assay detected a HBV strain that the commercial assay could not due to rare mutation(s).
HCC tissue from the original liver tumor, the lung tumor, and the serum specimens collected both at the
time of her HCC diagnosis and at the time of her lung metastasis were sent to the laboratory. DNA was
extracted from formalin-fixed liver or lung tissue blocks using the DNeasy Blood & Tissue Kit from Qiagen.
[7]
The extracted DNA was then subject to real time PCR .