Page 51 - Read Online
P. 51
Page 8 of 13 Spieler et al. Hepatoma Res 2019;5:4 I http://dx.doi.org/10.20517/2394-5079.2018.77
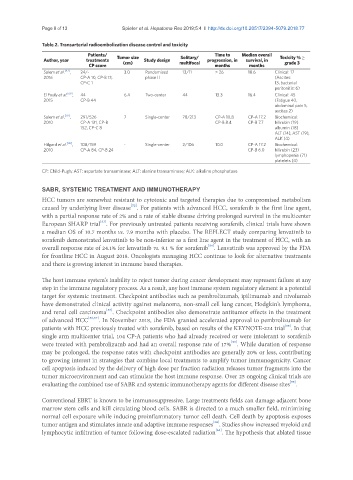
Table 2. Transarterial radioembolization disease control and toxicity
Patients/ Time to Median overall Toxicity % ≥
Author, year treatments Tumor size Study design Solitary/ progression, in survival, in
CP score (cm) multifocal months months grade 3
Salem et al. [47] , 24/- 3.0 Randomized 13/11 > 26 18.6 Clinical: 17
2016 CP-A 10, CP-B 13, phase II (Ascites:
CP-C 1 13, bacterial
peritonitis: 6)
El Fouly et al. [69] , 44 6.4 Two-center 44 13.3 16.4 Clinical: 45
2015 CP-B 44 (Fatigue 40,
abdominal pain 5,
ascites 2)
Salem et al. [70] , 291/526 7 Single-center 78/213 CP-A 10.8 CP-A 17.2 Biochemical:
2010 CP-A 131, CP-B CP-B 8.4 CP-B 7.7 bilirubin (19)
152, CP-C 8 albumin (18)
ALT (14), AST (19),
ALK (4)
Hilgard et al. [46] , 108/159 - Single-center 2/106 10.0 CP-A 17.2 Biochemical:
2010 CP-A 84, CP-B 24 CP-B 6.0 bilirubin (23)
lymphopenia (71)
platelets (4)
CP: Child-Pugh; AST: aspartate transaminase; ALT: alanine transaminase; ALK: alkaline phosphatase
SABR, SYSTEMIC TREATMENT AND IMMUNOTHERAPY
HCC tumors are somewhat resistant to cytotoxic and targeted therapies due to compromised metabolism
[52]
caused by underlying liver disease . For patients with advanced HCC, sorafenib is the first line agent,
with a partial response rate of 2% and a rate of stable disease driving prolonged survival in the multicenter
[53]
European SHARP trial . For previously untreated patients receiving sorafenib, clinical trials have shown
a median OS of 10.7 months vs. 7.9 months with placebo. The REFLECT study comparing lenvatinib to
sorafenib demonstrated lenvatinib to be non-inferior as a first line agent in the treatment of HCC, with an
[54]
overall response rate of 24.1% for lenvatinib vs. 9.1 % for sorafenib . Lenvatinib was approved by the FDA
for frontline HCC in August 2018. Oncologists managing HCC continue to look for alternative treatments
and there is growing interest in immune based therapies.
The host immune system’s inability to reject tumor during cancer development may represent failure at any
step in the immune regulatory process. As a result, any host immune system regulatory element is a potential
target for systemic treatment. Checkpoint antibodies such as pembrolizumab, ipilimumab and nivolumab
have demonstrated clinical activity against melanoma, non-small cell lung cancer, Hodgkin’s lymphoma,
[55]
and renal cell carcinoma . Checkpoint antibodies also demonstrate antitumor effects in the treatment
of advanced HCC [56,57] . In November 2018, the FDA granted accelerated approval to pembrolizumab for
[58]
patients with HCC previously treated with sorafenib, based on results of the KEYNOTE-224 trial . In that
single arm multicenter trial, 104 CP-A patients who had already received or were intolerant to sorafenib
[59]
were treated with pembrolizumb and had an overall response rate of 17% . While duration of response
may be prolonged, the response rates with checkpoint antibodies are generally 20% or less, contributing
to growing interest in strategies that combine local treatments to amplify tumor immunogenicity. Cancer
cell apoptosis induced by the delivery of high dose per fraction radiation releases tumor fragments into the
tumor microenvironment and can stimulate the host immune response. Over 25 ongoing clinical trials are
[55]
evaluating the combined use of SABR and systemic immunotherapy agents for different disease sites .
Conventional EBRT is known to be immunosuppressive. Large treatments fields can damage adjacent bone
marrow stem cells and kill circulating blood cells. SABR is directed to a much smaller field, minimizing
normal cell exposure while inducing proinflammatory tumor cell death. Cell death by apoptosis exposes
[60]
tumor antigen and stimulates innate and adaptive immune responses . Studies show increased myeloid and
[61]
lymphocytic infiltration of tumor following dose-escalated radiation . The hypothesis that ablated tissue