Page 46 - Read Online
P. 46
Spieler et al. Hepatoma Res 2019;5:4 I http://dx.doi.org/10.20517/2394-5079.2018.77 Page 3 of 13
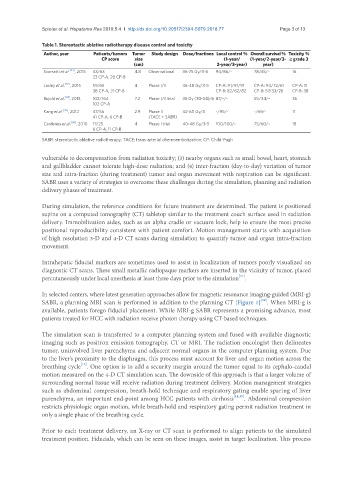
Table 1. Stereotactic ablative radiotherapy disease control and toxicity
Author, year Patients/tumors Tumor Study design Dose/fractions Local control % Overall survival % Toxicity %
CP score size (1-year/ (1-year/2-year/3- ≥ grade 3
(cm) 2-year/3-year) year)
Scorsetti et al. [43] , 2015 43/63 4.8 Observational 36-75 Gy/3-6 94/86/- 78/45/- 16
23 CP-A, 20 CP-B
Lasley et al. [52] , 2015 59/65 4 Phase I/II 36-48 Gy/3-5 CP-A: 91/91/91 CP-A: 94/72/61 CP-A: 11
38 CP-A, 21 CP-B CP-B: 82/82/82 CP-B: 57/33/26 CP-B: 38
Bujold et al. [41] , 2013 102/164 7.2 Phase I/II trial 36 Gy (30-54)/6 87/-/- 55/34/- 36
102 CP-A
Kang et al. [42] , 2012 47/56 2.9 Phase II 42-60 Gy/3 -/95/- -/69/- 11
41 CP-A, 6 CP-B (TACE + SABR)
Cárdenes et al. [68] , 2010 17/25 4 Phase I trial 40-48 Gy/3-5 100/100/- 75/60/- 18
6 CP-A, 11 CP-B
SABR: stereotactic ablative radiotherapy; TACE: trans arterial chemoembolization; CP: Child-Pugh
vulnerable to decompensation from radiation toxicity; (3) nearby organs such as small bowel, heart, stomach
and gallbladder cannot tolerate high-dose radiation; and (4) inter-fraction (day-to-day) variation of tumor
size and intra-fraction (during treatment) tumor and organ movement with respiration can be significant.
SABR uses a variety of strategies to overcome these challenges during the simulation, planning and radiation
delivery phases of treatment.
During simulation, the reference conditions for future treatment are determined. The patient is positioned
supine on a computed tomography (CT) tabletop similar to the treatment couch surface used in radiation
delivery. Immobilization aides, such as an alpha cradle or vacuum lock, help to ensure the most precise
positional reproducibility consistent with patient comfort. Motion management starts with acquisition
of high resolution 3-D and 4-D CT scans during simulation to quantify tumor and organ intra-fraction
movement.
Intrahepatic fiducial markers are sometimes used to assist in localization of tumors poorly visualized on
diagnostic CT scans. These small metallic radiopaque markers are inserted in the vicinity of tumor, placed
[11]
percutaneously under local anesthesia at least three days prior to the simulation .
In selected centers, where latest generation approaches allow for magnetic resonance imaging-guided (MRI-g)
[12]
SABR, a planning MRI scan is performed in addition to the planning CT [Figure 1] . When MRI-g is
available, patients forego fiducial placement. While MRI-g SABR represents a promising advance, most
patients treated for HCC with radiation receive photon therapy using CT-based techniques.
The simulation scan is transferred to a computer planning system and fused with available diagnostic
imaging such as positron emission tomography, CT or MRI. The radiation oncologist then delineates
tumor, uninvolved liver parenchyma and adjacent normal organs in the computer planning system. Due
to the liver’s proximity to the diaphragm, this process must account for liver and organ motion across the
[13]
breathing cycle . One option is to add a security margin around the tumor equal to its cephalo-caudal
motion measured on the 4-D CT simulation scan. The downside of this approach is that a larger volume of
surrounding normal tissue will receive radiation during treatment delivery. Motion management strategies
such as abdominal compression, breath-hold technique and respiratory gating enable sparing of liver
parenchyma, an important end-point among HCC patients with cirrhosis [14,15] . Abdominal compression
restricts physiologic organ motion, while breath-hold and respiratory gating permit radiation treatment in
only a single phase of the breathing cycle.
Prior to each treatment delivery, an X-ray or CT scan is performed to align patients to the simulated
treatment position. Fiducials, which can be seen on these images, assist in target localization. This process