Page 47 - Read Online
P. 47
Page 6 of 10 Chen et al. Hepatoma Res 2018;4:4 I http://dx.doi.org/10.20517/2394-5079.2017.50
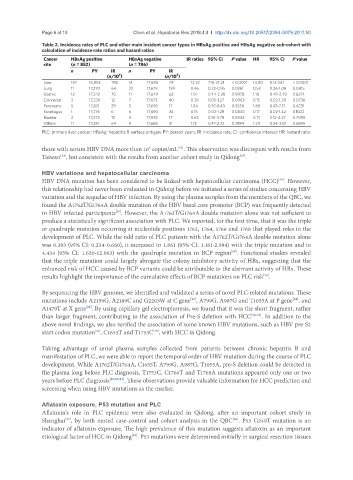
Table 2. Incidence rates of PLC and other main incident cancer types in HBsAg positive and HBsAg negative sub-cohort with
calculation of incidence rate ratios and hazard ratios
Cancer HBsAg positive HBsAg negative IR ratios 95% CI P value HR 95% CI P value
site (n = 852) (n = 786)
n PY IR n PY IR
(n/10 ) (n/10 )
5
5
Liver 187 16,853 1110 14 17,680 79 12.32 7.16-21.21 < 0.0001 14.00 8.13-24.1 < 0.0001
Lung 11 17,270 64 22 17,679 124 0.46 0.22-0.95 0.0361 0.53 0.26-1.08 0.0815
Gastric 12 17,242 70 11 17,649 62 1.01 0.44-2.28 0.9878 1.10 0.49-2.49 0.8214
Colorectal 2 17,230 12 7 17,673 40 0.26 0.05-1.27 0.0963 0.15 0.02-1.20 0.0736
Pancreatic 5 17,261 29 3 17,695 17 1.54 0.37-6.43 0.5558 1.80 0.43-7.51 0.4231
Esophagus 1 17,276 6 6 17,690 34 0.15 0.02-1.28 0.0830 0.17 0.02-1.42 0.1022
Bladder 2 17,272 12 3 17,692 17 0.62 0.10-3.78 0.5944 0.71 0.12-4.27 0.7099
Others 11 17,251 64 9 17,685 51 1.13 0.47-2.72 0.7894 1.29 0.54-3.12 0.5696
PLC: primary liver cancer; HBsAg: hepatitis B surface antigen; PY: person years; IR: incidence rate; CI: confidence interval; HR: hazard ratio
those with serum HBV DNA more than 10 copies/mL . This observation was discrepant with results from
6
[33]
Taiwan , but consistent with the results from another cohort study in Qidong .
[35]
[34]
HBV variations and hepatocellular carcinoma
HBV DNA mutation has been considered to be linked with hepatocellular carcinoma (HCC) . However,
[36]
this relationship had never been evaluated in Qidong before we initiated a series of studies concerning HBV
variation and the sequelae of HBV infection. By using the plasma samples from the members of the QBC, we
found the A1762T/G1764A double mutation of the HBV basal core promoter (BCP) was frequently detected
[16]
in HBV infected participants . However, the A1762T/G1764A double mutation alone was not sufficient to
produce a statistically significant association with PLC. We reported, for the first time, that it was the triple
or quadruple mutation occurring at nucleotide positions 1762, 1764, 1766 and 1768 that played roles in the
development of PLC. While the odd ratio of PLC patients with the A1762T/G1764A double mutation alone
was 0.393 (95% CI: 0.234-0.660), it increased to 1.861 (95% CI: 1.161-2.984) with the triple mutation and to
4.434 (95% CI: 1.630-12.063) with the quadruple mutation in BCP region . Functional studies revealed
[18]
that the triple mutation could largely abrogate the colony inhibitory activity of HBx, suggesting that the
enhanced risk of HCC caused by BCP variants could be attributable to the aberrant activity of HBx. These
results highlight the importance of the cumulative effects of BCP mutations on PLC risk .
[19]
By sequencing the HBV genome, we identified and validated a series of novel PLC-related mutations. These
mutations include A2159G, A2189C and G2203W at C gene , A799G, A987G and T1055A at P gene , and
[23]
[24]
A1479T at X gene . By using capillary gel electrophoresis, we found that it was the short fragment, rather
[18]
than larger fragment, contributing to the association of Pre-S deletion with HCC [26,27] . In addition to the
above novel findings, we also verified the association of some known HBV mutations, such as HBV pre-S2
[21]
start codon mutation , C1653T and T1753C , with HCC in Qidong.
[19]
Taking advantage of serial plasma samples collected from patients between chronic hepatitis B and
manifestation of PLC, we were able to report the temporal order of HBV mutation during the course of PLC
development. While A1762T/G1764A, C1653T, A799G, A987G, T1055A, pre-S deletion could be detected in
the plasma long before PLC diagnosis, T1753C, C1766T and T1768A mutations appeared only one or two
years before PLC diagnosis [18,20,23] . These observations provide valuable information for HCC prediction and
screening when using HBV mutations as the marker.
Aflatoxin exposure, P53 mutation and PLC
Aflatoxin’s role in PLC epidemic were also evaluated in Qidong, after an important cohort study in
Shanghai , by both nested case-control and cohort analysis in the QBC . P53 G249T mutation is an
[38]
[37]
indicator of aflatoxin exposure. The high prevalence of this mutation suggests aflatoxin as an important
etiological factor of HCC in Qidong . P53 mutations were determined initially in surgical resection tissues
[39]