Page 240 - Read Online
P. 240
Page 8 of 15 Missale et al. Hepatoma Res 2018;4:22 I http://dx.doi.org/10.20517/2394-5079.2018.72
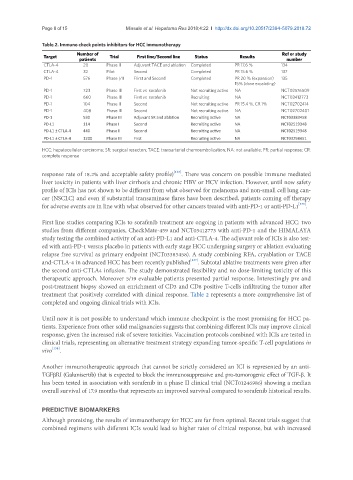
Table 2. Immuno check points inhibitors for HCC immunotherapy
Target Number of Trial First line/Second line Status Results Ref or study
patients number
CTLA-4 20 Phase II Adjuvant TACE and ablation Completed PR 17.6 % 134
CTLA-4 32 Pilot Second Completed PR 15.6 % 137
PD-1 576 Phase I/II FIirst and Second Completed PR 20 % (expansion) 135
15% (dose excalating)
PD-1 723 Phase III First vs. sorafenib Not recruiting active NA NCT02576509
PD-1 660 Phase III First vs. sorafenib Recruiting NA NCT03412773
PD-1 104 Phase II Second Not recruiting active PR 15.4 %, CR 1% NCT02702414
PD-1 408 Phase III Second Not recruiting active NA NCT02702401
PD-1 530 Phase III Adjuvant SR and ablation Recruiting active NA NCT03383458
PD-L1 114 Phase I Second Recruiting active NA NCT02519348
PD-L1 ± CTLA-4 440 Phase II Second Recruiting active NA NCT02519348
PD-L1 ± CTLA-4 1200 Phase III First Recruiting active NA NCT03298451
HCC: hepatocellular carcinoma; SR: surgical resecton; TACE: transarterial chemoembolization; NA: not available; PR: partial response; CR:
complete response
response rate of 18.2% and acceptable safety profile) [135] . There was concern on possible immune mediated
liver toxicity in patients with liver cirrhosis and chronic HBV or HCV infection. However, until now safety
profile of ICIs has not shown to be different from what observed for melanoma and non-small cell lung can-
cer (NSCLC) and even if substantial transaminase flares have been described, patients coming off therapy
[136]
for adverse events are in line with what observed for other cancers treated with anti-PD-1 or anti-PD-L1 .
First line studies comparing ICIs to sorafenib treatment are ongoing in patients with advanced HCC: two
studies from different companies, CheckMate-459 and NCT03412773 with anti-PD-1 and the HIMALAYA
study testing the combined activity of an anti-PD-L1 and anti-CTLA-4. The adjuvant role of ICIs is also test-
ed with anti-PD-1 versus placebo in patients with early stage HCC undergoing surgery or ablation evaluating
relapse free survival as primary endpoint (NCT03383458). A study combining RFA, cryablation or TACE
and-CTLA-4 in advanced HCC has been recently published [137] . Subtotal ablative treatments were given after
the second anti-CTLA4 infusion. The study demonstrated feasibility and no dose-limiting toxicity of this
therapeutic approach. Moreover 5/19 evaluable patients presented partial response. Interestingly pre and
post-treatment biopsy showed an enrichment of CD3 and CD8 positive T-cells infiltrating the tumor after
treatment that positively correlated with clinical response. Table 2 represents a more comprehensive list of
completed and ongoing clinical trials with ICIs.
Until now it is not possible to understand which immune checkpoint is the most promising for HCC pa-
tients. Experience from other solid malignancies suggests that combining different ICIs may improve clinical
response, given the increased risk of severe toxicities. Vaccination protocols combined with ICIs are tested in
clinical trials, representing an alternative treatment strategy expanding tumor-specific T-cell populations in
vivo [138] .
Another immunotherapeutic approach that cannot be strictly considered an ICI is represented by an anti-
TGFbRI (Galunisertib) that is expected to block the immunosuppressive and pro-tumorogenic effect of TGF-β. It
has been tested in association with sorafenib in a phase II clinical trial (NCT01246986) showing a median
overall survival of 17.9 months that represents an improved survival compared to sorafenib historical results.
PREDICTIVE BIOMARKERS
Although promising, the results of immunotherapy for HCC are far from optimal. Recent trials suggest that
combined regimens with different ICIs would lead to higher rates of clinical response, but with increased