Page 38 - Read Online
P. 38
Page 4 of 13 Kwee et al. Hepatoma Res 2021;7:8 I http://dx.doi.org/10.20517/2394-5079.2020.124
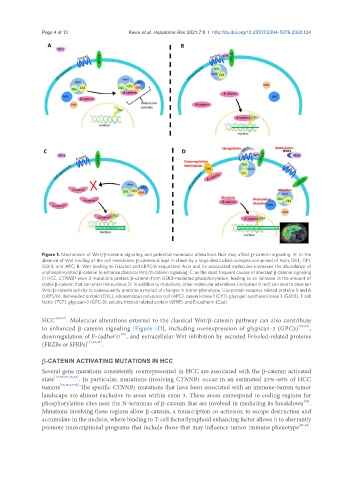
Figure 1. Mechanism of Wnt/b-catenin signaling and potential molecular alterations that may affect b-catenin signaling. A: In the
absence of Wnt binding at the cell membrane, b-catenin is kept in check by a large destruction complex comprised of Axin, DVL, CK1,
GSK3, and APC; B: Wnt binding to Frizzled and LRP5/6 sequesters Axin and its associated molecules increases the abundance of
unphosphorylated b-catenin to enhance classical Wnt/b-catenin signaling; C: as the most frequent causes of aberrant b-catenin signaling
in HCC, CTNNB1 exon 3 mutations protect b-catenin from GSK3-mediated phosphorylation, leading to an increase in the amount of
stable b-catenin that can enter the nucleus; D: in addition to mutations, other molecular alterations (indicated in red) can lead to aberrant
Wnt/b-catenin activity to consequently promote a myriad of changes in tumor phenotype. Lipoprotein receptor related proteins 5 and 6
(LRP5/6), dishevelled protein (DVL), adenomatous polyposis coli (APC), casein kinase 1 (CK1), glycogen synthase kinase 3 (GSK3), T cell
factor (TCF), glypican-3 (GPC-3), soluble frizzled related protein (SFRP), and E-cadherin (Cad)
HCC [35,37] . Molecular alterations external to the classical Wnt/b-catenin pathway can also contribute
to enhanced b-catenin signaling [Figure 1D], including overexpression of glypican-3 (GPC3) [38,39] ,
[40]
downregulation of E-cadherin , and extracellular Wnt inhibition by secreted Frizzled-related proteins
(FRZBs or SFRPs) [11,12,41] .
b-CATENIN ACTIVATING MUTATIONS IN HCC
Several gene mutations consistently overrepresented in HCC are associated with the b-catenin activated
state [12,33,35,36,42] . In particular, mutations involving CTNNB1 occur in an estimated 25%-40% of HCC
tumors [12,16,23,36] The specific CTNNB1 mutations that have been associated with an immune-barren tumor
landscape are almost exclusive to areas within exon 3. These areas correspond to coding regions for
[34]
phosphorylation sites near the N-terminus of b-catenin that are involved in mediating its breakdown .
Mutations involving these regions allow b-catenin, a transcription co-activator, to escape destruction and
accumulate in the nucleus, where binding to T-cell factor/lymphoid enhancing factor allows it to aberrantly
promote transcriptional programs that include those that may influence tumor immune phenotype [43-45] .