Page 10 - Read Online
P. 10
Page 4 of 6 Best et al. Hepatoma Res 2021;7:32 https://dx.doi.org/10.20517/2394-5079.2021.52
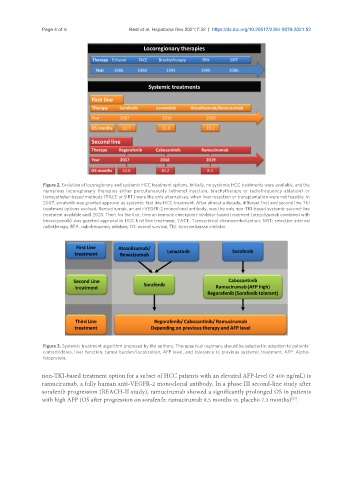
Figure 2. Evolution of locoregionary and systemic HCC treatment options. Initially, no systemic HCC treatments were available, and the
numerous locoregionary therapies either percutaneously (ethanol injection, brachytherapy or radiofrequency ablation) or
transcatheter-based methods (TACE or SIRT) were the only alternatives, when liver resection or transplantation were not feasible. In
2007, sorafenib was granted approval as systemic first line HCC treatment. After almost a decade, different first and second line TKI
treatment options evolved. Ramucirumab, an anti-VEGFR-2 monoclonal antibody, was the only non-TKI-based systemic second-line
treatment available until 2020. Then, for the first, time an immune checkpoint-inhibitor-based treatment (atezolizumab combined with
bevacizumab) was granted approval in HCC first line treatment. TACE: Transarterial chemoembolization; SIRT: selective internal
radiotherapy; RFA: radiofrequency ablation; OS: overall survival; TKI: tyrosine kinase inhibitor.
Figure 3. Systemic treatment algorithm proposed by the authors. Therapeutical regimens should be selected in adaption to patients’
comorbidities, liver function, tumor burden/localization, AFP level, and tolerance to previous systemic treatment. AFP: Alpha-
fetoprotein.
non-TKI-based treatment option for a subset of HCC patients with an elevated AFP-level (≥ 400 ng/mL) is
ramucirumab, a fully human anti-VEGFR-2 monoclonal antibody. In a phase III second-line study after
sorafenib progression (REACH-II study), ramucirumab showed a significantly prolonged OS in patients
with high AFP (OS after progression on sorafenib: ramucirumab 8.5 months vs. placebo 7.3 months) .
[23]