Page 43 - Read Online
P. 43
Page 6 of 9 Dai et al. Hepatoma Res 2020;6:16 I http://dx.doi.org/10.20517/2394-5079.2019.40
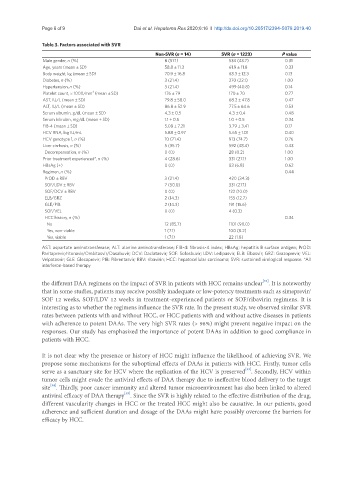
Table 3. Factors associated with SVR
Non-SVR (n = 14) SVR (n = 1223) P value
Male gender, n (%) 8 (57.1) 534 (43.7) 0.31
Age, years (mean ± SD) 58.8 ± 11.3 61.9 ± 11.8 0.33
Body weight, kg (mean ± SD) 70.9 ± 16.8 63.3 ± 12.3 0.13
Diabetes, n (%) 3 (21.4) 270 (22.1) 1.00
Hypertension, n (%) 3 (21.4) 499 (40.8) 0.14
3
Platelet count, × 1000/mm (mean ± SD) 176 ± 79 170 ± 70 0.77
AST, IU/L (mean ± SD) 79.8 ± 58.0 68.2 ± 47.8 0.47
ALT, IU/L (mean ± SD) 86.8 ± 52.9 77.5 ± 64.6 0.53
Serum albumin, g/dL (mean ± SD) 4.3 ± 0.5 4.3 ± 0.4 0.48
Serum bilirubin, mg/dL (mean ± SD) 1.1 ± 0.5 1.0 ± 0.5 0.34
FIB-4 (mean ± SD) 5.08 ± 7.21 3.79 ± 3.41 0.17
HCV RNA, log IU/mL 5.88 ± 0.97 5.65 ± 1.01 0.40
HCV genotype 1, n (%) 10 (71.4) 913 (74.7) 0.76
Liver cirrhosis, n (%) 5 (35.7) 592 (48.4) 0.43
Decompensation, n (%) 0 (0) 28 (0.2) 1.00
Prior treatment experienced*, n (%) 4 (28.6) 331 (27.1) 1.00
HBsAg (+) 0 (0) 83 (6.8) 0.62
Regimen, n (%) 0.44
PrOD ± RBV 3 (21.4) 420 (34.3)
SOF/LDV ± RBV 7 (50.0) 331 (27.1)
SOF/DCV ± RBV 0 (0) 122 (10.0)
ELB/GRZ 2 (14.3) 155 (12.7)
GLE/PIB 2 (14.3) 191 (15.6)
SOF/VEL 0 (0) 4 (0.3)
HCC history, n (%) 0.34
No 12 (85.7) 1101 (90.0)
Yes, non-viable 1 (7.1) 100 (8.2)
Yes, viable 1 (7.1) 22 (1.8)
AST: aspartate aminotransferase; ALT: alanine aminotransferase; FIB-4: fibrosis-4 index; HBsAg: hepatitis B surface antigen; PrOD:
Paritaprevir/ritonavir/Ombitasvir/Dasabuvir; DCV: Daclatasvir; SOF: Sofosbuvir; LDV: Ledipasvir; ELB: Elbasvir; GRZ: Grazoprevir; VEL:
Velpatasvir; GLE: Glecaprevir; PIB: Pibrentasvir; RBV: ribavirin; HCC: hepatocellular carcinoma; SVR: sustained virological response. *All
interferon-based therapy
[32]
the different DAA regimens on the impact of SVR in patients with HCC remains unclear . It is noteworthy
that in some studies, patients may receive possibly inadequate or low-potency treatments such as simeprevir/
SOF 12 weeks, SOF/LDV 12 weeks in treatment-experienced patients or SOF/ribavirin regimens. It is
interesting as to whether the regimens influence the SVR rate. In the present study, we observed similar SVR
rates between patients with and without HCC, or HCC patients with and without active diseases in patients
with adherence to potent DAAs. The very high SVR rates (> 98%) might prevent negative impact on the
responses. Our study has emphasized the importance of potent DAAs in addition to good compliance in
patients with HCC.
It is not clear why the presence or history of HCC might influence the likelihood of achieving SVR. We
propose some mechanisms for the suboptimal effects of DAAs in patients with HCC. Firstly, tumor cells
[33]
serve as a sanctuary site for HCV where the replication of the HCV is preserved . Secondly, HCV within
tumor cells might evade the antiviral effects of DAA therapy due to ineffective blood delivery to the target
[34]
site . Thirdly, poor cancer immunity and altered tumor microenvironment has also been linked to altered
[35]
antiviral efficacy of DAA therapy . Since the SVR is highly related to the effective distribution of the drug,
different vascularity changes in HCC or the treated HCC might also be causative. In our patients, good
adherence and sufficient duration and dosage of the DAAs might have possibly overcome the barriers for
efficacy by HCC.