Page 42 - Read Online
P. 42
Dai et al. Hepatoma Res 2020;6:16 I http://dx.doi.org/10.20517/2394-5079.2019.40 Page 5 of 9
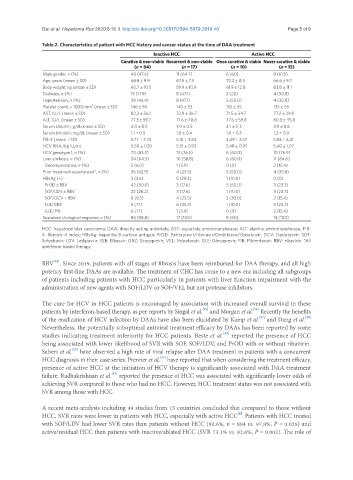
Table 2. Characteristics of patient with HCC history and cancer status at the time of DAA treatment
Inactive HCC Active HCC
Curative & non-viable Recurrent & non-viable Once curative & viable Never curative & viable
(n = 84) (n = 17) (n = 10) (n = 13)
Male gender, n (%) 40 (47.6) 11 (64.7) 6 (60) 8 (61.5)
Age, years (mean ± SD) 68.8 ± 9.9 67.8 ± 7.3 70.2 ± 8.3 66.6 ± 9.7
Body weight, kg (mean ± SD) 60.7 ± 10.5 59.9 ± 10.9 61.9 ± 12.8 63.8 ± 11.1
Diabetes, n (%) 15 (17.9) 8 (47.1) 2 (20) 4 (30.8)
Hypertension, n (%) 39 (46.4) 8 (47.1) 5 (50.0) 4 (30.8)
3
Platelet count, × 1000/mm (mean ± SD) 146 ± 94 140 ± 53 152 ± 55 135 ± 65
AST, IU/L (mean ± SD) 82.3 ± 56.1 72.9 ± 36.7 71.5 ± 34.7 77.2 ± 29.8
ALT, IU/L (mean ± SD) 77.3 ± 59.7 71.6 ± 48.8 77.6 ± 59.8 80.3 ± 45.8
Serum albumin, g/dL(mean ± SD) 4.0 ± 0.5 3.9 ± 0.5 4.1 ± 0.3 3.9 ± 0.6
Serum bilirubin, mg/dL (mean ± SD) 1.1 + 0.5 1.0 ± 0.4 1.0 + 0.5 1.3 + 0.9
FIB-4 (mean ± SD) 5.77 ± 3.73 5.31 ± 3.84 4.49 ± 2.47 5.93 ± 4.41
HCV RNA, log IU/mL 5.50 ± 1.00 5.51 ± 0.93 5.48 ± 0.97 5.40 ± 1.07
HCV genotype 1, n (%) 70 (83.3) 13 (76.5) 6 (60.0) 10 (76.9)
Liver cirrhosis, n (%) 54 (64.3) 10 (58.8) 6 (60.0) 11 (84.6)
Decompensation, n (%) 5 (6.0) 1 (5.9) 0 (0) 2 (15.4)
Prior treatment experienced*, n (%) 36 (42.9) 4 (23.5) 5 (50.0) 4 (30.8)
HBsAg (+) 3 (3.6) 5 (29.4) 1 (10.0) 0 (0)
PrOD ± RBV 42 (50.0) 3 (17.6) 5 (50.0) 3 (23.3)
SOF/LDV ± RBV 22 (26.2) 3 (17.6) 1 (10.0) 3 (23.3)
SOF/DCV ± RBV 8 (9.5) 4 (23.5) 3 (30.0) 2 (15.4)
ELB/GRZ 6 (7.1) 6 (35.3) 1 (10.0) 3 (23.3)
GLE/PIB 6 (7.1) 1 (5.9) 0 (0) 2 (15.4)
Sustained virological response, n (%) 83 (98.8) 17 (100) 9 (90) 13 (100)
HCC: hepatocellular carcinoma; DAA: directly acting antivirals; AST: aspartate aminotransferase; ALT: alanine aminotransferase; FIB-
4: fibrosis-4 index; HBsAg: hepatitis B surface antigen; PrOD: Paritaprevir/ritonavir/Ombitasvir/Dasabuvir; DCV: Daclatasvir; SOF:
Sofosbuvir; LDV: Ledipasvir; ELB: Elbasvir; GRZ: Grazoprevir; VEL: Velpatasvir; GLE: Glecaprevir; PIB: Pibrentasvir; RBV: ribavirin. *All
interferon-based therapy
[24]
RBV . Since 2019, patients with all stages of fibrosis have been reimbursed for DAA therapy, and all high
potency first-line DAAs are available. The treatment of CHC has come to a new era including all subgroups
of patients including patients with HCC particularly in patients with liver function impairment with the
administration of new agents with SOF/LDV or SOF/VEL but not protease inhibitors.
The cure for HCV in HCC patients is encouraged by association with increased overall survival in these
[26]
[25]
patients by interferon-based therapy, as per reports by Singal et al. and Morgan et al. Recently the benefits
[28]
[27]
of the eradication of HCV infection by DAAs have also been elucidated by Kamp et al. and Dang et al.
Nevertheless, the potentially suboptimal antiviral treatment efficacy by DAAs has been reported by some
[29]
studies indicating treatment inferiority for HCC patients. Beste et al. reported the presence of HCC
being associated with lower likelihood of SVR with SOF, SOF/LDV, and PrOD with or without ribavirin.
[10]
Saberi et al. have observed a high rate of viral relapse after DAA treatment in patients with a concurrent
[30]
HCC diagnosis in their case series. Prenner et al. have reported that when considering the treatment efficacy,
presence of active HCC at the initiation of HCV therapy is significantly associated with DAA treatment
[31]
failure. Radhakrishnan et al. reported the presence of HCC was associated with significantly lower odds of
achieving SVR compared to those who had no HCC. However, HCC treatment status was not associated with
SVR among those with HCC.
A recent meta-analysis including 49 studies from 15 countries concluded that compared to those without
[9]
HCC, SVR rates were lower in patients with HCC, especially with active HCC . Patients with HCC treated
with SOF/LDV had lower SVR rates than patients without HCC (92.6%, n = 884 vs. 97.8%, P = 0.026) and
active/residual HCC than patients with inactive/ablated HCC (SVR 73.1% vs. 92.6%, P = 0.002). The role of