Page 23 - Read Online
P. 23
Burlone et al. Hepatoma Res 2020;6:3 I http://dx.doi.org/10.20517/2394-5079.2019.37 Page 5 of 10
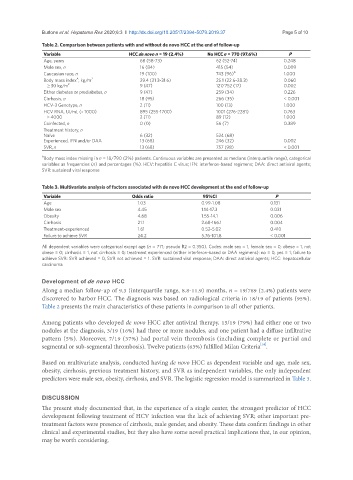
Table 2. Comparison between patients with and without de novo HCC at the end of follow-up
Variable HCC de novo n = 19 (2.4%) No HCC n = 770 (97.6%) P
Age, years 68 (58-73) 62 (52-74) 0.248
Male sex, n 16 (84) 415 (54) 0.009
Caucasian race, n 19 (100) 743 (96) A 1.000
Body mass index , kg/m 2 29.4 (21.3-31.6) 25.1 (22.6-28.3) 0.060
A
≥ 30 kg/m 2 9 (47) 127/752 (17) 0.002
Either diabetes or prediabetes, n 9 (47) 259 (34) 0.226
Cirrhosis, n 18 (95) 266 (35) < 0.001
HCV-3 Genotype, n 2 (11) 100 (13) 1.000
HCV RNA, UI/mL (× 1000) 895 (255-1700) 1001 (276-2281) 0.763
> 4000 2 (11) 89 (12) 1.000
Coinfected, n 0 (0) 56 (7) 0.389
Treatment history, n
Naïve 6 (32) 524 (68)
Experienced, IFN and/or DAA 13 (68) 246 (32) 0.002
SVR, n 13 (68) 757 (98) < 0.001
A Body mass index missing in n = 18/790 (2%) patients. Continuous variables are presented as medians (interquartile range), categorical
variables as frequencies (n) and percentages (%). HCV: hepatitis C virus; IFN: interferon-based regimens; DAA: direct antiviral agents;
SVR: sustained viral response
Table 3. Multivariate analysis of factors associated with de novo HCC development at the end of follow-up
Variable Odds ratio 95%CI P
Age 1.03 0.99-1.08 0.131
Male sex 4.45 1.14-17.3 0.031
Obesity 4.68 1.55-14.1 0.006
Cirrhosis 21.1 2.68-166.1 0.004
Treatment-experienced 1.61 0.52-5.02 0.410
Failure to achieve SVR 24.2 5.76-101.8 < 0.001
All dependent variables were categorical except age (n = 771; pseudo R2 = 0.350). Codes: male sex = 1, female sex = 0; obese = 1, not
obese = 0; cirrhosis = 1, not cirrhosis = 0; treatment experienced (either interferon-based or DAA regimens): no = 0, yes = 1; failure to
achieve SVR: SVR achieved = 0, SVR not achieved = 1. SVR: sustained viral response; DAA: direct antiviral agents; HCC: hepatocellular
carcinoma
Development of de novo HCC
Along a median follow-up of 9.3 (interquartile range, 8.8-11.9) months, n = 19/789 (2.4%) patients were
discovered to harbor HCC. The diagnosis was based on radiological criteria in 18/19 of patients (95%).
Table 2 presents the main characteristics of these patients in comparison to all other patients.
Among patients who developed de novo HCC after antiviral therapy, 15/19 (79%) had either one or two
nodules at the diagnosis, 3/19 (16%) had three or more nodules, and one patient had a diffuse infiltrative
pattern (5%). Moreover, 7/19 (37%) had portal vein thrombosis (including complete or partial and
[14]
segmental or sub-segmental thrombosis). Twelve patients (63%) fulfilled Milan Criteria .
Based on multivariate analysis, conducted having de novo HCC as dependent variable and age, male sex,
obesity, cirrhosis, previous treatment history, and SVR as independent variables, the only independent
predictors were male sex, obesity, cirrhosis, and SVR. The logistic regression model is summarized in Table 3.
DISCUSSION
The present study documented that, in the experience of a single center, the strongest predictor of HCC
development following treatment of HCV infection was the lack of achieving SVR; other important pre-
treatment factors were presence of cirrhosis, male gender, and obesity. These data confirm findings in other
clinical and experimental studies, but they also have some novel practical implications that, in our opinion,
may be worth considering.