Page 28 - Read Online
P. 28
Page 10 of 13 da Fonseca et al. Hepatoma Res 2019;5:37 I http://dx.doi.org/10.20517/2394-5079.2019.012
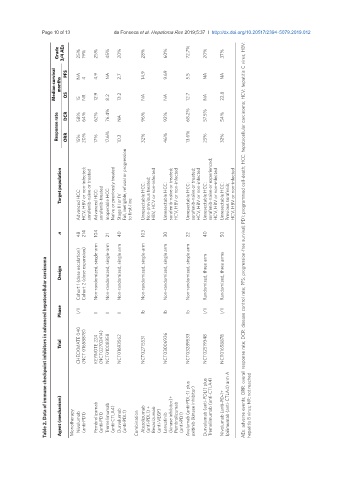
Grade 3/4 AEs 25% 19% 25% 45% 20% 28% 60% 72.7% 20% 37%
Median survival months PFS OS NA 15 4 NR 4.9 12.9 NA 8.2 2.7 13.2 14.9 NA 9.69 NA 5.5 12.7 NA NA NA 22.8
Response rate DCR ORR 58% 15% 64% 20% 62% 17% 76.4% 17.6% NA 10.3 96% 32% 92% 46% 68.2% 13.6% 57.5% 25% 54% 32%
Target population HCV, HBV or non-infected; sorafenib-naïve or treated Naïve or previously treated Fail, ineligible, refusal or progression HBV, HCV or non-infected sorafenib-naïve or treated; HCV, HBV or non-infected sorafenib-naïve or treated; HCV, HBV or non-infected sorafenib-naïve or experienced; HCV, HBV or non-infected HCV, HBV or non-infected
Advanced HCC: Advanced HCC: sorafenib-treated Inoperable HCC: Stage III or IV to first-line Unresectable HCC: Non-previous treated; Unresectable HCC: Unresectable HCC: Unresectable HCC: Unresectable HCC: Previous sorafenib;
n 48 214 104 21 40 103 30 22 40 50 AEs: adverse events; ORR: overall response rate; DCR: disease control rate; PFS: progression-free survival; PD1: programmed cell-death; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HBV:
Cohort 1 (dose escalation) Randomized, three arm Randomized, three arms
Table 2. Data of immune checkpoint inhibitors in advanced hepatocellular carcinoma
Design Cohort 2 (dose expansion) Non-randomized, single-arm Non-randomized, single-arm Non-randomized, single arm Non-randomized, single-arm Non-randomized, single arm Non-randomized, single arm
Phase I/II II II II Ib Ib Ib I/II I/II
CHECKMATE 040 (NCT 01658878) KEYNOTE 224 (NCT02702414) NCT01008358 NCT01693562 NCT02715531 NCT03006926 NCT03289533 NCT02519348 NCT01658878
Trial
Agent (mechanism) Monotherapy Nivolumab (anti-PD1) Pembrolizumab (anti-PD1) Tremelimumab (anti-CTLA4) Durvalumab (anti-PDL1) Combination Atezolizumab (anti-PDL1) + Bevacizumab (anti-VEGF) Lenvatinib (kinase inhibitor)+ Pembrolizumab (anti-PD1) Avelumab (anti-PDL-1) plus axitinib (kinase inhibitor) Durvalumab (anti-PDL1) plus Tremelimumab (anti-CTLA4) Nivolumab (anti-PD-1+ Ipilimu