Page 26 - Read Online
P. 26
Page 8 of 13 da Fonseca et al. Hepatoma Res 2019;5:37 I http://dx.doi.org/10.20517/2394-5079.2019.012
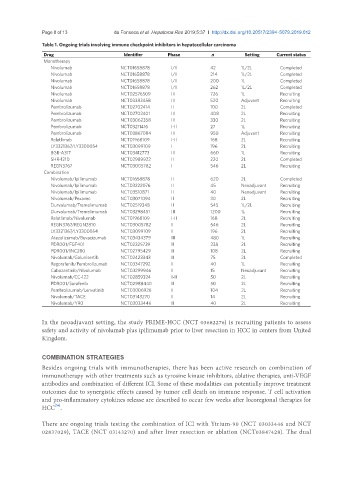
Table 1. Ongoing trials involving immune checkpoint inhibitors in hepatocellular carcinoma
Drug Identifier Phase n Setting Current status
Monotherapy
Nivolumab NCT01658878 I/II 42 1L/2L Completed
Nivolumab NCT01658878 I/II 214 1L/2L Completed
Nivolumab NCT01658878 I/II 200 1L Completed
Nivolumab NCT01658878 I/II 262 1L/2L Completed
Nivolumab NCT02576509 III 726 1L Recruiting
Nivolumab NCT03383458 III 520 Adjuvant Recruiting
Pembrolizumab NCT02702414 II 100 2L Completed
Pembrolizumab NCT02702401 III 408 2L Recruiting
Pembrolizumab NCT03062358 III 330 2L Recruiting
Pembrolizumab NCT03211416 I-II 27 1L Recruiting
Pembrolizumab NCT03867084 III 950 Adjuvant Recruiting
Relatlimab NCT01968109 I-II 168 2L Recruiting
LY3321367/LY3300054 NCT03099109 I 196 2L Recruiting
BGB-A317 NCT03412773 III 660 1L Recruiting
SHR-1210 NCT02989922 II 220 2L Completed
REGN3767 NCT03005782 I 546 2L Recruiting
Combination
Nivolumab/Ipilimumab NCT01658878 II 620 2L Completed
Nivolumab/Ipilimumab NCT03222076 II 45 Neoadjuvant Recruiting
Nivolumab/Ipilimumab NCT03510871 II 40 Neoadjuvant Recruiting
Nivolumab/Pexavec NCT03071094 II 30 2L Recruiting
Durvalumab/Tremelimumab NCT02519348 II 545 1L/2L Recruiting
Durvalumab/Tremelimumab NCT03298451 III 1200 1L Recruiting
Relatlimab/Nivolumab NCT01968109 I-II 168 2L Recruiting
REGN3767/REGN2810 NCT03005782 I 546 2L Recruiting
LY3321367/LY3300054 NCT03099109 I 196 2L Recruiting
Atezolizumab/Bevacizumab NCT03434379 III 480 1L Recruiting
PDR001/FGF401 NCT02325739 II 238 2L Recruiting
PDR001/INC280 NCT02795429 II 108 2L Recruiting
Nivolumab/Galunisertib NCT02423343 II 75 2L Completed
Regorafenib/Pembrolizumab NCT03347292 I 40 1L Recruiting
Cabozantinib/Nivolumab NCT03299946 I 15 Neoadjuvant Recruiting
Nivolumab/CC-122 NCT02859324 I-II 50 2L Recruiting
PDR001/Sorafenib NCT02988440 II 50 2L Recruiting
Pembrolizumab/Lenvatinib NCT03006926 I 104 2L Recruiting
Nivolumab/TACE NCT03143270 I 14 2L Recruiting
Nivolumab/Y90 NCT03033446 II 40 2L Recruiting
In the neoadjuvant setting, the study PRIME-HCC (NCT 03682276) is recruiting patients to assess
safety and activity of nivolumab plus ipilimumab prior to liver resection in HCC in centers from United
Kingdom.
COMBINATION STRATEGIES
Besides ongoing trials with immunotherapies, there has been active research on combination of
immunotherapy with other treatments such as tyrosine kinase inhibitors, ablative therapies, anti-VEGF
antibodies and combination of different ICI. Some of these modalities can potentially improve treatment
outcomes due to synergistic effects caused by tumor cell death on immune response. T cell activation
and pro-inflammatory cytokines release are described to occur few weeks after locoregional therapies for
[59]
HCC .
There are ongoing trials testing the combination of ICI with Ytrium-90 (NCT 03033446 and NCT
02837029), TACE (NCT 03143270) and after liver resection or ablation (NCT03847428). The dual