Page 52 - Read Online
P. 52
Reyngold et al. Hepatoma Res 2018;4:49 I http://dx.doi.org/10.20517/2394-5079.2018.84 Page 7 of 10
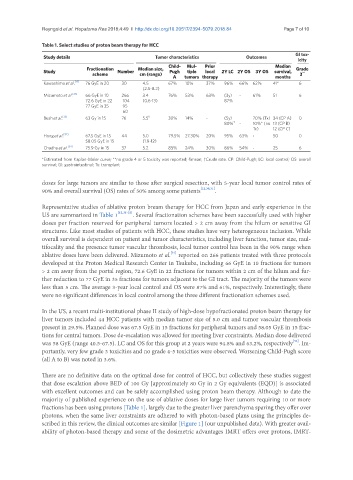
Table 1. Select studies of proton beam therapy for HCC
GI tox-
Study details Tumor characteristics Outcomes
icity
Child- Mul- Prior Median
Fractionation Median size, Grade
Study Number Pugh tiple local 2Y LC 2Y OS 3Y OS survival, **
scheme cm (range) 3
A tumors therapy months
Kawashima et al. [12] 76 GyE in 20 30 4.5 67% 10% 37% 96% 66% 62% 41* 6
(2.5-8.2)
Mizumoto et al. [31] 66 GyE in 10 266 3.4 76% 53% 63% (3y) - 61% 51 6
72.6 GyE in 22 104 (0.6-13) 87%
77 GyE in 35 95
60
Bush et al. [33] 63 Gy in 15 76 5.5 § 30% 14% - (5y) 70% (Tx) 34 (CP A) 0
80% † - 10%* (no 13 (CP B)
Tx) 12 (CP C)
Hong et al. [32] 67.5 GyE in 15 44 5.0 79.5% 27.30% 20% 95% 63% - 50 0
58.05 GyE in 15 (1.9-12)
Chadha et al. [34] 75.9 Gy in 15 37 5.2 85% 24% 30% 86% 54% - 25 6
*Estimated from Kaplan-Meier curve; **no grade 4 or 5 toxicity was reported; §mean; †Crude rate. CP: Child-Pugh; LC: local control; OS: overall
survival; GI: gastrointestinal; Tx: transplant
doses for large tumors are similar to those after surgical resection, with 5-year local tumor control rates of
90% and overall survival (OS) rates of 50% among some patients [12,30,31] .
Representative studies of ablative proton bream therapy for HCC from Japan and early experience in the
US are summarized in Table 1 [12,31-33] . Several fractionation schemes have been successfully used with higher
doses per fraction reserved for peripheral tumors located > 2 cm away from the hilum or sensitive GI
structures. Like most studies of patients with HCC, these studies have very heterogeneous inclusion. While
overall survival is dependent on patient and tumor characteristics, including liver function, tumor size, mul-
tifocality and the presence tumor vascular thrombosis, local tumor control has been in the 90% range when
[31]
ablative doses have been delivered. Mizumoto et al. reported on 266 patients treated with three protocols
developed at the Proton Medical Research Center in Tsukuba, including 66 GyE in 10 fractions for tumors
> 2 cm away from the portal region, 72.6 GyE in 22 fractions for tumors within 2 cm of the hilum and fur-
ther reduction to 77 GyE in 35 fractions for tumors adjacent to the GI tract. The majority of the tumors were
less than 5 cm. The average 3-year local control and OS were 87% and 61%, respectively. Interestingly, there
were no significant differences in local control among the three different fractionation schemes used.
In the US, a recent multi-institutional phase II study of high-dose hypofractionated proton beam therapy for
liver tumors included 44 HCC patients with median tumor size of 5.0 cm and tumor vascular thrombosis
present in 29.5%. Planned dose was 67.5 GyE in 15 fractions for peripheral tumors and 58.05 GyE in 15 frac-
tions for central tumors. Dose de-escalation was allowed for meeting liver constraints. Median dose delivered
[32]
was 58 GyE (range 40.5-67.5). LC and OS for this group at 2 years were 94.8% and 63.2%, respectively . Im-
portantly, very few grade 3 toxicities and no grade 4-5 toxicities were observed. Worsening Child-Pugh score
(all A to B) was noted in 3.6%.
There are no definitive data on the optimal dose for control of HCC, but collectively these studies suggest
that dose escalation above BED of 100 Gy [approximately 80 Gy in 2 Gy equivalents (EQD)] is associated
with excellent outcomes and can be safely accomplished using proton beam therapy. Although to date the
majority of published experience on the use of ablative doses for large liver tumors requiring 10 or more
fractions has been using protons [Table 1], largely due to the greater liver parenchyma sparing they offer over
photons, when the same liver constraints are adhered to with photon-based plans using the principles de-
scribed in this review, the clinical outcomes are similar [Figure 1] (our unpublished data). With greater avail-
ability of photon-based therapy and some of the dosimetric advantages IMRT offers over protons, IMRT-