Page 28 - Read Online
P. 28
Gavriilidis et al. Hepatoma Res 2023;9:23 https://dx.doi.org/10.20517/2394-5079.2023.26 Page 5 of 8
Figure 3. Summary of published consensus guidelines on the indications for resection of intrahepatic cholangiocarcinoma.
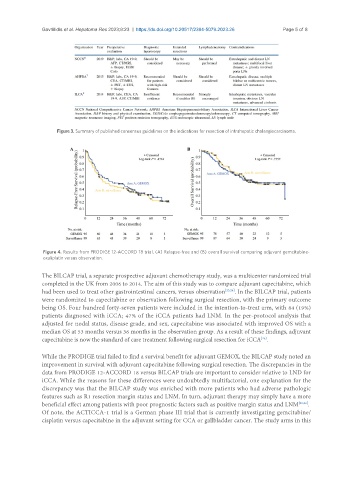
Figure 4. Results from PRODIGE 12-ACCORD 18 trial. (A) Relapse-free and (B) overall survival comparing adjuvant gemcitabine-
oxaliplatin versus observation.
The BILCAP trial, a separate prospective adjuvant chemotherapy study, was a multicenter randomized trial
completed in the UK from 2006 to 2014. The aim of this study was to compare adjuvant capecitabine, which
had been used to treat other gastrointestinal cancers, versus observation [23,24] . In the BILCAP trial, patients
were randomized to capecitabine or observation following surgical resection, with the primary outcome
being OS. Four hundred forty-seven patients were included in the intention-to-treat arm, with 84 (19%)
patients diagnosed with iCCA; 47% of the iCCA patients had LNM. In the per-protocol analysis that
adjusted for nodal status, disease grade, and sex, capecitabine was associated with improved OS with a
median OS at 53 months versus 36 months in the observation group. As a result of these findings, adjuvant
capecitabine is now the standard of care treatment following surgical resection for iCCA .
[25]
While the PRODIGE trial failed to find a survival benefit for adjuvant GEMOX, the BILCAP study noted an
improvement in survival with adjuvant capecitabine following surgical resection. The discrepancies in the
data from PRODIGE 12-ACCORD 18 versus BILCAP trials are important to consider relative to LND for
iCCA. While the reasons for these differences were undoubtedly multifactorial, one explanation for the
discrepancy was that the BILCAP study was enriched with more patients who had adverse pathologic
features such as R1 resection margin status and LNM. In turn, adjuvant therapy may simply have a more
beneficial effect among patients with poor prognostic factors such as positive margin status and LNM [20,26] .
Of note, the ACTICCA-1 trial is a German phase III trial that is currently investigating gemcitabine/
cisplatin versus capecitabine in the adjuvant setting for CCA or gallbladder cancer. The study arms in this