Page 177 - Read Online
P. 177
Page 6 of 20 Gim et al. Hepatoma Res 2023;9:51 https://dx.doi.org/10.20517/2394-5079.2023.90
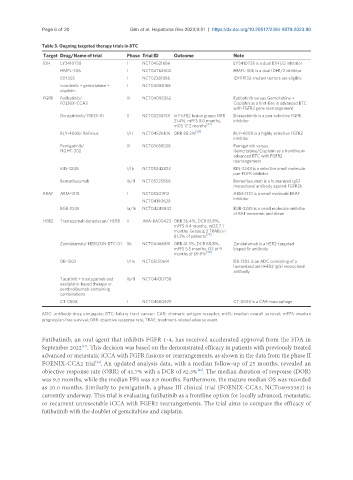
Table 3. Ongoing targeted therapy trials in BTC
Target Drug/Name of trial Phase Trial ID Outcome Note
IDH LY3410738 I NCT04521686 LY3410738 is a dual IDH1/2 inhibitor
HMPL-306 I NCT04762602 HMPL-306 is a dual IDH1/2 inhibitor
IDH305 I NCT02381886 IDH1R132-mutant tumors are eligible
ivosidenib + gemcitabine + I NCT04088188
cisplatin
FGFR Futibatinib/ III NCT04093362 Futibatinib versus Gemcitabine +
FOENIX-CCA3 Cisplatin as a first-line in advanced BTC
with FGFR2 gene rearrangement
Derazabtinib/ FIDES-01 II NCT03230318 In FGFR2 fusion group: ORR Derazabtinib is a pan-selective FGFR
21.4%, mPFS 8.0 months, inhibitor
[27]
mOS 17.2 months
RLY-4008/ ReFocus I/II NCT04526106 ORR 88.2% [28] RLY-4008 is a highly selective FGFR2
inhibitor
Pemigatinib/ III NCT03656536 Pemigatinib versus
FIGHT-302 Gemcitabine/Cisplatin as a frontline in
advanced BTC with FGFR2
rearrangement
KIN-3248 I/Ib NCT05242822 KIN-3248 is a selective small molecule
pan-FGFR inhibitor
Bemarituzumab Ib/II NCT05325866 Bemarituzumab is a humanized IgG1
monoclonal antibody against FGFR2b
BRAF ABM-1310 I NCT05501912 ABM-1310 is a small molecule BRAF
inhibitor
NCT04190628
BGB-3245 Ia/Ib NCT04249843 BGB-3245 is a small molecule inhibitor
of RAF monomer and dimer
HER2 Trastuzumab deruxtecan/ HERB II JMA-IIA00423 ORR 36.4%, DCR 81.8%,
mPFS 4.4 months, mOS 7.1
months. Grade ≥ 3 TRAEs in
[29]
81.3% of patients
Zanidatamab/ HERIZON-BTC-01 IIb NCT04466891 ORR 41.3%, DCR 68.8%, Zanidatamab is a HER2-targeted
mPFS 5.5 months, OS at 9 bispecific antibody
months of 69.9% [30]
DB-1303 I/IIa NCT05150691 DB-1303 is an ADC consisting of a
humanized anti-HER2 IgG1 monoclonal
antibody
Tucatinib + trastuzumab and Ib/II NCT04430738
oxaliplatin-based therapy or
pembrolizumab-containing
combinations
CT-0508 I NCT04660929 CT-0508 is a CAR-macrophage
ADC: antibody-drug conjugate; BTC: biliary tract cancer; CAR: chimeric antigen receptor; mOS: median overall survival; mPFS: median
progression-free survival; ORR: objective response rate; TRAE: treatment-related adverse event.
Futibatinib, an oral agent that inhibits FGFR 1-4, has received accelerated approval from the FDA in
September 2022 . This decision was based on the demonstrated efficacy in patients with previously treated
[44]
advanced or metastatic iCCA with FGFR fusions or rearrangements, as shown in the data from the phase II
FOENIX-CCA2 trial . An updated analysis data, with a median follow-up of 25 months, revealed an
[45]
[46]
objective response rate (ORR) of 41.7% with a DCR of 82.5% . The median duration of response (DOR)
was 9.5 months, while the median PFS was 8.9 months. Furthermore, the mature median OS was recorded
as 20.0 months. Similarly to pemigatinib, a phase III clinical trial (FOENIX-CCA3, NCT04093362) is
currently underway. This trial is evaluating futibatinib as a frontline option for locally advanced, metastatic,
or recurrent unresectable iCCA with FGFR2 rearrangements. The trial aims to compare the efficacy of
futibatinib with the doublet of gemcitabine and cisplatin.