Page 176 - Read Online
P. 176
Gim et al. Hepatoma Res 2023;9:51 https://dx.doi.org/10.20517/2394-5079.2023.90 Page 5 of 20
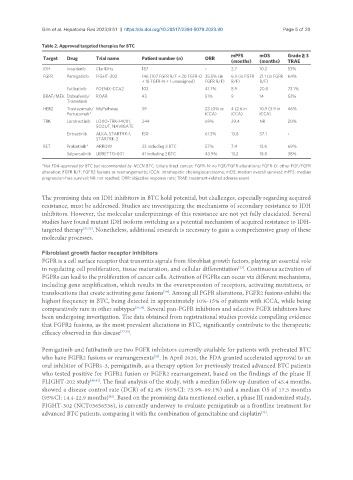
Table 2. Approved targeted therapies for BTC
mPFS mOS Grade ≥ 3
Target Drug Trial name Patient number (n) ORR
(months) (months) TRAE
IDH Ivosidenib ClarIDHy 187 - 2.7 10.3 53%
FGFR Pemigatinib FIGHT-202 146 (107 FGFR R/F + 20 FGFR-O 35.5% (in 6.9 (in FGFR 21.1 (in FGFR 64%
+ 18 FGFR-N + 1 unassigned) FGFR R/F) R/F) R/F)
Futibatinib FOENIX-CCA2 103 41.7% 8.9 20.0 73.1%
BRAF/MEK Dabrafenib/ ROAR 43 51% 9 14 53%
Trametinib
HER2 Trastuzumab/ MyPathway 39 23 (0% in 4 (2.6 in 10.9 (3.9 in 46%
Pertuzumab* iCCA) iCCA) iCCA)
TRK Larotrectinib LOXO-TRK-14001, 244 69% 29.4 NR 20%
SCOUT, NAVIGATE
Entrectinib ALKA, STARTRK-1, 150 61.3% 13.8 37.1 -
STARTRK-2
RET Pralsetinib* ARROW 23 including 3 BTC 57% 7.4 13.6 69%
Selpercatinib LIBRETTO-001 41 including 2 BTC 43.9% 13.2 18.0 38%
*Not FDA-approved for BTC but recommended by NCCN. BTC: biliary tract cancer; FGFR-N: no FGF/FGFR alterations; FGFR-O: other FGF/FGFR
alteration; FGFR R/F: FGFR2 fusions or rearrangements; iCCA: intrahepatic cholangiocarcinoma; mOS: median overall survival; mPFS: median
progression-free survival; NR: not reached; ORR: objective response rate; TRAE: treatment-related adverse event.
The promising data on IDH inhibitors in BTC hold potential, but challenges, especially regarding acquired
resistance, must be addressed. Studies are investigating the mechanisms of secondary resistance to IDH
inhibitors. However, the molecular underpinnings of this resistance are not yet fully elucidated. Several
studies have found mutant IDH isoform switching as a potential mechanism of acquired resistance to IDH-
targeted therapy [31,32] . Nonetheless, additional research is necessary to gain a comprehensive grasp of these
molecular processes.
Fibroblast growth factor receptor inhibitors
FGFR is a cell surface receptor that transmits signals from fibroblast growth factors, playing an essential role
in regulating cell proliferation, tissue maturation, and cellular differentiation . Continuous activation of
[33]
FGFRs can lead to the proliferation of cancer cells. Activation of FGFRs can occur via different mechanisms,
including gene amplification, which results in the overexpression of receptors, activating mutations, or
translocations that create activating gene fusions . Among all FGFR alterations, FGFR2 fusions exhibit the
[34]
highest frequency in BTC, being detected in approximately 10%-15% of patients with iCCA, while being
comparatively rare in other subtypes [35,36] . Several pan-FGFR inhibitors and selective FGFR inhibitors have
been undergoing investigation. The data obtained from registrational studies provide compelling evidence
that FGFR2 fusions, as the most prevalent alterations in BTC, significantly contribute to the therapeutic
efficacy observed in this disease [37,38] .
Pemigatinib and futibatinib are two FGFR inhibitors currently available for patients with pretreated BTC
who have FGFR2 fusions or rearrangements . In April 2020, the FDA granted accelerated approval to an
[39]
oral inhibitor of FGFR1-3, pemigatinib, as a therapy option for previously treated advanced BTC patients
who tested positive for FGFR2 fusion or FGFR2 rearrangement, based on the findings of the phase II
FLIGHT-202 study [40,41] . The final analysis of the study, with a median follow-up duration of 45.4 months,
showed a disease control rate (DCR) of 82.4% (95%CI: 73.9%-89.1%) and a median OS of 17.5 months
(95%CI: 14.4-22.9 months) . Based on the promising data mentioned earlier, a phase III randomized study,
[42]
FIGHT-302 (NCT03656536), is currently underway to evaluate pemigatinib as a frontline treatment for
[43]
advanced BTC patients, comparing it with the combination of gemcitabine and cisplatin .