Page 175 - Read Online
P. 175
Page 4 of 20 Gim et al. Hepatoma Res 2023;9:51 https://dx.doi.org/10.20517/2394-5079.2023.90
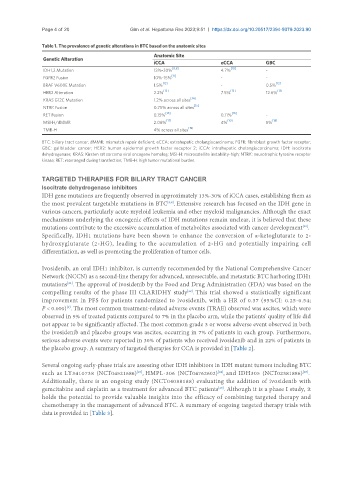
Table 1. The prevalence of genetic alterations in BTC based on the anatomic sites
Anatomic Site
Genetic Alteration
iCCA eCCA GBC
IDH1,2 Mutation 13%-30% [8,9] 4.7% [10] -
[11]
FGFR2 Fusion 10%-15% - -
BRAF V600E Mutation 1.5% [12] - 0.5% [12]
[13] [13] [13]
HER2 Alteration 2.2% 7.5% 12.6%
[14]
KRAS G12C Mutation 1.2% across all sites
[15]
NTRK Fusion 0.75% across all sites
[16] [16]
RET Fusion 0.15% 0.11% -
MSI-H/dMMR 2.06% [17] 4% [10] 5% [18]
[19]
TMB-H 4% across all sites
BTC: biliary tract cancer; dMMR: mismatch repair deficient; eCCA: extrahepatic cholangiocarcinoma; FGFR: fibroblast growth factor receptor;
GBC: gallbladder cancer; HER2: human epidermal growth factor receptor 2; iCCA: intrahepatic cholangiocarcinoma; IDH: isocitrate
dehydrogenase; KRAS: Kirsten rat sarcoma viral oncogene homolog; MSI-H: microsatellite instability-high; NTRK: neurotrophic tyrosine receptor
kinase; RET: rearranged during transfection; TMB-H: high tumor mutational burden.
TARGETED THERAPIES FOR BILIARY TRACT CANCER
Isocitrate dehydrogenase inhibitors
IDH gene mutations are frequently observed in approximately 13%-30% of iCCA cases, establishing them as
the most prevalent targetable mutations in BTC . Extensive research has focused on the IDH gene in
[8,9]
various cancers, particularly acute myeloid leukemia and other myeloid malignancies. Although the exact
mechanisms underlying the oncogenic effects of IDH mutations remain unclear, it is believed that these
mutations contribute to the excessive accumulation of metabolites associated with cancer development .
[20]
Specifically, IDH1 mutations have been shown to enhance the conversion of α-ketoglutarate to 2-
hydroxyglutarate (2-HG), leading to the accumulation of 2-HG and potentially impairing cell
differentiation, as well as promoting the proliferation of tumor cells.
Ivosidenib, an oral IDH1 inhibitor, is currently recommended by the National Comprehensive Cancer
Network (NCCN) as a second-line therapy for advanced, unresectable, and metastatic BTC harboring IDH1
mutations . The approval of ivosidenib by the Food and Drug Administration (FDA) was based on the
[21]
compelling results of the phase III CLARIDHY study . This trial showed a statistically significant
[22]
improvement in PFS for patients randomized to ivosidenib, with a HR of 0.37 (95%CI: 0.25-0.54;
P < 0.000) . The most common treatment-related adverse events (TRAE) observed was ascites, which were
[9]
observed in 9% of treated patients compared to 7% in the placebo arm, while the patients' quality of life did
not appear to be significantly affected. The most common grade 3 or worse adverse event observed in both
the ivosidenib and placebo groups was ascites, occurring in 7% of patients in each group. Furthermore,
serious adverse events were reported in 30% of patients who received ivosidenib and in 22% of patients in
the placebo group. A summary of targeted therapies for CCA is provided in [Table 2].
Several ongoing early-phase trials are assessing other IDH inhibitors in IDH mutant tumors including BTC
such as LY3410738 (NCT04521686) , HMPL-306 (NCT04762602) , and IDH305 (NCT02381886) .
[25]
[23]
[24]
Additionally, there is an ongoing study (NCT04088188) evaluating the addition of ivosidenib with
gemcitabine and cisplatin as a treatment for advanced BTC patients . Although it is a phase I study, it
[26]
holds the potential to provide valuable insights into the efficacy of combining targeted therapy and
chemotherapy in the management of advanced BTC. A summary of ongoing targeted therapy trials with
data is provided in [Table 3].