Page 63 - Read Online
P. 63
Rutter et al. Extracell Vesicles Circ Nucleic Acids 2023;4:90-106 https://dx.doi.org/10.20517/evcna.2023.04 Page 94
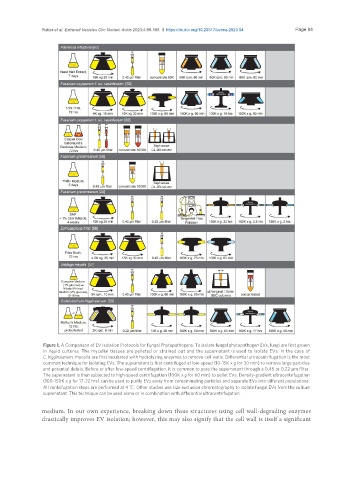
Figure 1. A Comparison of EV Isolation Protocols for Fungal Phytopathogens. To isolate fungal phytopathogen EVs, fungi are first grown
in liquid cultures. The mycelial tissues are pelleted or strained out and the supernatant is used to isolate EVs. In the case of
C. higginsianum, mycelia are first incubated with hydrolyzing enzymes to remove cell walls. Differential ultracentrifugation is the most
common technique for isolating EVs. The supernatant is first centrifuged at low-speed (10-15K x g for 30 min) to remove large particles
and potential debris. Before or after low-speed centrifugation, it is common to pass the supernatant through a 0.45 or 0.22 µm filter.
The supernatant is then subjected to high-speed centrifugation (100K x g for 60 min) to pellet EVs. Density-gradient ultracentrifugation
(100-150K x g for 17-22 hrs) can be used to purify EVs away from contaminating particles and separate EVs into different populations.
All centrifugation steps are performed at 4 ℃. Other studies use size exclusion chromatography to isolate fungal EVs from the culture
supernatant. This technique can be used alone or in combination with differential ultracentrifugation.
medium. In our own experience, breaking down these structures using cell wall-degrading enzymes
drastically improves EV isolation; however, this may also signify that the cell wall is itself a significant