Page 188 - Read Online
P. 188
Asao et al. Extracell Vesicles Circ Nucleic Acids 2023;4:461-85 https://dx.doi.org/10.20517/evcna.2023.37 Page 7
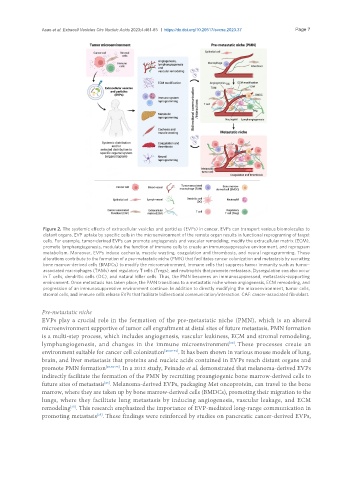
Figure 2. The systemic effects of extracellular vesicles and particles (EVPs) in cancer. EVPs can transport various biomolecules to
distant organs. EVP uptake by specific cells in the microenvironment of the remote organ results in functional reprograming of target
cells. For example, tumor-derived EVPs can promote angiogenesis and vascular remodeling, modify the extracellular matrix (ECM),
promote lymphangiogenesis, modulate the function of immune cells to create an immunosuppressive environment, and reprogram
metabolism. Moreover, EVPs induce cachexia, muscle wasting, coagulation and thrombosis, and neural reprogramming. These
alterations contribute to the formation of a pre-metastatic niche (PMN) that facilitates cancer colonization and metastasis by recruiting
bone marrow-derived cells (BMDCs) to modify the microenvironment, immune cells that suppress tumor immunity such as tumor-
associated macrophages (TAMs) and regulatory T cells (Tregs), and neutrophils that promote metastasis. Dysregulation can also occur
in T cells, dendritic cells (DC), and natural killer cells. Thus, the PMN becomes an immunosuppressed, metastasis-supporting
environment. Once metastasis has taken place, the PMN transitions to a metastatic niche where angiogenesis, ECM remodeling, and
progression of an immunosuppressive environment continue. In addition to directly modifying the microenvironment, tumor cells,
stromal cells, and immune cells release EVPs that facilitate bidirectional communication/interaction. CAF: cancer-associated fibroblast.
Pre-metastatic niche
EVPs play a crucial role in the formation of the pre-metastatic niche (PMN), which is an altered
microenvironment supportive of tumor cell engraftment at distal sites of future metastasis. PMN formation
is a multi-step process, which includes angiogenesis, vascular leakiness, ECM and stromal remodeling,
lymphangiogenesis, and changes in the immune microenvironment . These processes create an
[68]
environment suitable for cancer cell colonization [49,69-71] . It has been shown in various mouse models of lung,
brain, and liver metastasis that proteins and nucleic acids contained in EVPs reach distant organs and
promote PMN formation [25,72-75] . In a 2012 study, Peinado et al. demonstrated that melanoma-derived EVPs
indirectly facilitate the formation of the PMN by recruiting proangiogenic bone marrow-derived cells to
[25]
future sites of metastasis . Melanoma-derived EVPs, packaging Met oncoprotein, can travel to the bone
marrow, where they are taken up by bone marrow-derived cells (BMDCs), promoting their migration to the
lungs, where they facilitate lung metastasis by inducing angiogenesis, vascular leakage, and ECM
remodeling . This research emphasized the importance of EVP-mediated long-range communication in
[25]
promoting metastasis . These findings were reinforced by studies on pancreatic cancer-derived EVPs,
[25]