Page 59 - Read Online
P. 59
Page 8 of 16 Mu et al. Energy Mater 2022;2:200043 https://dx.doi.org/10.20517/energymater.2022.57
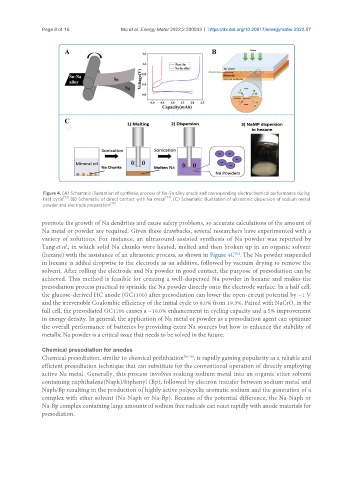
Figure 4. (A) Schematic illustration of synthesis process of Na-Sn alloy anode and corresponding electrochemical performance during
first cycle [73] . (B) Schematic of direct contact with Na metal [74] . (C) Schematic illustration of ultrasonic dispersion of sodium metal
powder and electrode preparation [75] .
promote the growth of Na dendrites and cause safety problems, so accurate calculations of the amount of
Na metal or powder are required. Given these drawbacks, several researchers have experimented with a
variety of solutions. For instance, an ultrasound-assisted synthesis of Na powder was reported by
Tang et al., in which solid Na chunks were heated, melted and then broken up in an organic solvent
(hexane) with the assistance of an ultrasonic process, as shown in Figure 4C . The Na powder suspended
[75]
in hexane is added dropwise to the electrode as an additive, followed by vacuum drying to remove the
solvent. After rolling the electrode and Na powder in good contact, the purpose of presodiation can be
achieved. This method is feasible for creating a well-dispersed Na powder in hexane and makes the
presodiation process practical to sprinkle the Na powder directly onto the electrode surface. In a half cell,
the glucose-derived HC anode (GC1100) after presodiation can lower the open-circuit potential by ~1 V
and the irreversible Coulombic efficiency of the initial cycle to 8.0% from 19.3%. Paired with NaCrO in the
2
full cell, the presodiated GC1100 causes a ~10.0% enhancement in cycling capacity and a 5% improvement
in energy density. In general, the application of Na metal or powder as a presodiation agent can optimize
the overall performance of batteries by providing extra Na sources but how to enhance the stability of
metallic Na powder is a critical issue that needs to be solved in the future.
Chemical presodiation for anodes
Chemical presodiation, similar to chemical prelithiation [76-79] , is rapidly gaining popularity as a reliable and
efficient presodiation technique that can substitute for the conventional operation of directly employing
active Na metal. Generally, this process involves soaking sodium metal into an organic ether solvent
containing naphthalene(Naph)/biphenyl (Bp), followed by electron transfer between sodium metal and
Naph/Bp resulting in the production of highly active polycyclic aromatic sodium and the generation of a
complex with ether solvent (Na-Naph or Na-Bp). Because of the potential difference, the Na-Naph or
Na-Bp complex containing large amounts of sodium free radicals can react rapidly with anode materials for
presodiation.