Page 185 - Read Online
P. 185
Page 6 of 9 He et al. Chem Synth 2023;3:35 https://dx.doi.org/10.20517/cs.2023.14
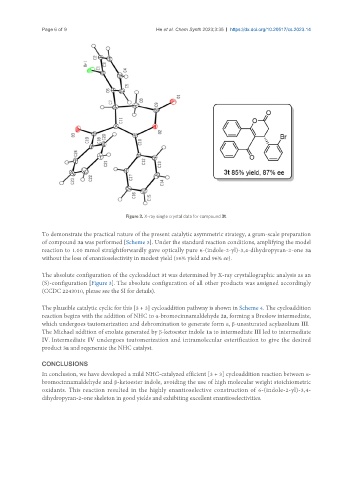
Figure 3. X-ray single crystal data for compound 3t.
To demonstrate the practical nature of the present catalytic asymmetric strategy, a gram-scale preparation
of compound 3a was performed [Scheme 3]. Under the standard reaction conditions, amplifying the model
reaction to 1.00 mmol straightforwardly gave optically pure 6-(indole-2-yl)-3,4-dihydropyran-2-one 3a
without the loss of enantioselectivity in modest yield (56% yield and 96% ee).
The absolute configuration of the cycloadduct 3t was determined by X-ray crystallographic analysis as an
(S)-configuration [Figure 3]. The absolute configuration of all other products was assigned accordingly
(CCDC 2243010, please see the SI for details).
The plausible catalytic cyclic for this [3 + 3] cycloaddition pathway is shown in Scheme 4. The cycloaddition
reaction begins with the addition of NHC to α-bromocinnamaldehyde 2a, forming a Breslow intermediate,
which undergoes tautomerization and debromination to generate form α, β-unsaturated acylazolium III.
The Michael addition of enolate generated by β-ketoester indole 1a to intermediate III led to intermediate
IV. Intermediate IV undergoes tautomerization and intramolecular esterification to give the desired
product 3a and regenerate the NHC catalyst.
CONCLUSIONS
In conclusion, we have developed a mild NHC-catalyzed efficient [3 + 3] cycloaddition reaction between α-
bromocinnamaldehyde and β-ketoester indole, avoiding the use of high molecular weight stoichiometric
oxidants. This reaction resulted in the highly enantioselective construction of 6-(indole-2-yl)-3,4-
dihydropyran-2-one skeleton in good yields and exhibiting excellent enantioselectivities.