Page 175 - Read Online
P. 175
Guo et al. Chem Synth 2023;3:34 https://dx.doi.org/10.20517/cs.2023.04 Page 7 of 11
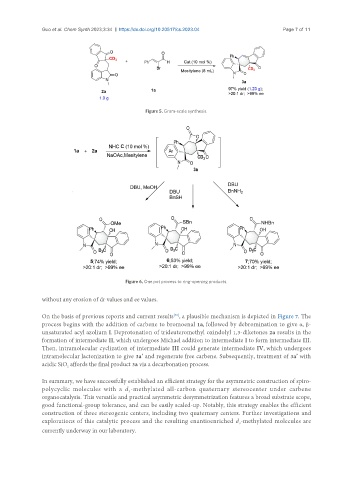
Figure 5. Gram-scale synthesis.
Figure 6. One pot process to ring-opening products.
without any erosion of dr values and ee values.
[46]
On the basis of previous reports and current results , a plausible mechanism is depicted in Figure 7. The
process begins with the addition of carbene to bromoenal 1a, followed by debromination to give α, β-
unsaturated acyl azolium I. Deprotonation of trideuteromethyl oxindolyl 1,3-diketones 2a results in the
formation of intermediate II, which undergoes Michael addition to intermediate I to form intermediate III.
Then, intramolecular cyclization of intermediate III could generate intermediate IV, which undergoes
intramolecular lactonization to give 3a’ and regenerate free carbene. Subsequently, treatment of 3a’ with
acidic SiO affords the final product 3a via a decarbonation process.
2
In summary, we have successfully established an efficient strategy for the asymmetric construction of spiro-
polycyclic molecules with a d -methylated all-carbon quaternary stereocenter under carbene
3
organocatalysis. This versatile and practical asymmetric desymmetrization features a broad substrate scope,
good functional-group tolerance, and can be easily scaled-up. Notably, this strategy enables the efficient
construction of three stereogenic centers, including two quaternary centers. Further investigations and
explorations of this catalytic process and the resulting enantioenriched d -methylated molecules are
3
currently underway in our laboratory.