Page 174 - Read Online
P. 174
Page 6 of 11 Guo et al. Chem Synth 2023;3:34 https://dx.doi.org/10.20517/cs.2023.04
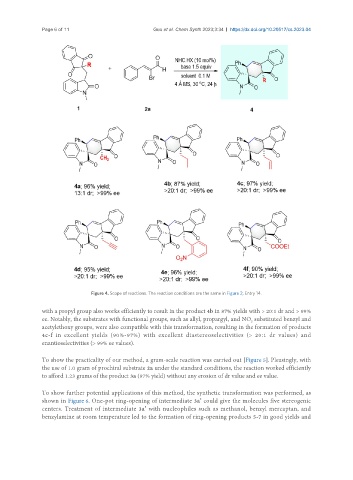
Figure 4. Scope of reactions. The reaction conditions are the same in Figure 2, Entry 14.
with a propyl group also works efficiently to result in the product 4b in 87% yields with > 20:1 dr and > 99%
ee. Notably, the substrates with functional groups, such as allyl, propargyl, and NO substituted benzyl and
2
acetylethoxy groups, were also compatible with this transformation, resulting in the formation of products
4c-f in excellent yields (90%-97%) with excellent diastereoselectivities (> 20:1 dr values) and
enantioselectivities (> 99% ee values).
To show the practicality of our method, a gram-scale reaction was carried out [Figure 5]. Pleasingly, with
the use of 1.0 gram of prochiral substrate 2a under the standard conditions, the reaction worked efficiently
to afford 1.23 grams of the product 3a (97% yield) without any erosion of dr value and ee value.
To show further potential applications of this method, the synthetic transformation was performed, as
shown in Figure 6. One-pot ring-opening of intermediate 3a’ could give the molecules five stereogenic
centers. Treatment of intermediate 3a’ with nucleophiles such as methanol, benzyl mercaptan, and
benzylamine at room temperature led to the formation of ring-opening products 5-7 in good yields and