Page 172 - Read Online
P. 172
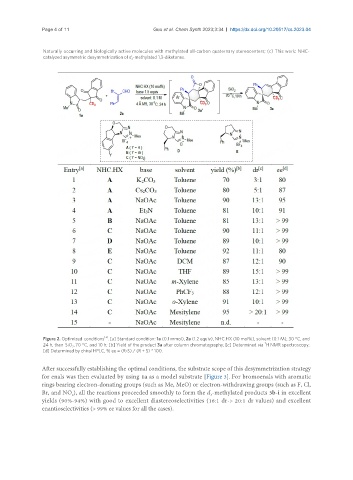
Page 4 of 11 Guo et al. Chem Synth 2023;3:34 https://dx.doi.org/10.20517/cs.2023.04
Naturally occurring and biologically active molecules with methylated all-carbon quaternary stereocenters; (c) This work: NHC-
catalyzed asymmetric desymmetrization of d -methylated 1,3-diketones.
3
[a]
Figure 2. Optimized conditions . [a] Standard condition: 1a (0.1 mmol), 2a (1.2 equiv), NHC.HX (10 mol%), solvent (0.1 M), 30 °C, and
1
24 h, then SiO , 70 °C, and 10 h; [b] Yield of the product 3a after column chromatography; [c] Determined via H NMR spectroscopy;
2
[d] Determined by chiral HPLC, % ee = (R-S) / (R + S) * 100.
After successfully establishing the optimal conditions, the substrate scope of this desymmetrization strategy
for enals was then evaluated by using 1a as a model substrate [Figure 3]. For bromoenals with aromatic
rings bearing electron-donating groups (such as Me, MeO) or electron-withdrawing groups (such as F, Cl,
Br, and NO ), all the reactions proceeded smoothly to form the d -methylated products 3b-i in excellent
3
2
yields (90%-94%) with good to excellent diastereoselectivities (16:1 dr-> 20:1 dr values) and excellent
enantioselectivities (> 99% ee values for all the cases).