Page 11 - Read Online
P. 11
Cheng et al. Chem Synth 2023;3:13 https://dx.doi.org/10.20517/cs.2022.43 Page 5 of 13
Table 1. UV-Vis absorption data for 1-5 at 298 K
-1
-1
Complex Medium Absorption λ/nm (ε/dm mol cm )
3
t
[Pt( Bu tpy)(C≡CC H C≡CH)]OTf (1) CH CN 285 (52,030), 322 (18,620), 338 (19,130), 420 (6,660)
3
3 6 4
t
[Pt( Bu tpy)(C≡CC H C HN C H )]OTf (2) CH CN 287 (49,290), 305 sh (35,150), 324 sh (16,330), 338 (15,980), 401 sh (15,980), 434
3
6
4 2
3 6
3
13
(5,560)
t
[PF-{N C H-C H C≡CPt( Bu tpy)} ](OTf) CH CN 288 (71,290), 308 sh (55,900), 341 (37,020), 383 (48,560), 445 sh (8,010)
3 2 6 4 3 2 2n 3
(3) a
t
[PFP-{N C H-C H C≡CPt( Bu tpy)} ](OTf) CH CN 287 (69,780), 308 sh (57,730), 341 (38,350), 374 (41,140), 451 sh (8,190)
3 2 6 4 3 2 2n 3
(4) a
t
[PFT-{N C H-C H C≡CPt( Bu tpy)} ](OTf) CH CN 289 (64,250), 308 sh (50,700), 340 sh (25,560), 409 (33,250), 466 sh (8,320)
3 2 6 4 3 2 2n 3
(5) a
a
The molar extinction coefficients of the metallopolymers were approximated per repeating unit.
Table 2. Emission data for 1-5
Complex Medium (T/K) λ /nm (τ /μs) Φ lum a
em
o
t -2b
[Pt( Bu tpy)(C≡CC H C≡CH)]OTf (1) CH CN (298) 596 (1.09) 5.0 × 10
3
3 6 4
t
[Pt( Bu tpy)(C≡CC H -C HN C H )]OTf (2) CH CN (298) 630 (0.14) 8.5 × 10 -3b
3
4
3
6
2
3 6
13
c
t CH CN (298) 416 (< 0.1), 671 (0.14) 1.2 × 10 -3d
[PF-{N C H-C H C≡C-Pt( Bu tpy)} ](OTf) (3) 3
2n
2
3
4
6
3 2
2.6 × 10 -3b
t c -4d
[PFP-{N C H-C H C≡C-Pt( Bu tpy)} ](OTf) (4) CH CN (298) 417 (< 0.1), 673 (0.70) 9.0 × 10
3
3 2 6 4 3 2 2n -2b
1.4 × 10
c
t
[PFT-{N C H-C H C≡C-Pt( Bu tpy)} ](OTf) (5) CH CN (298) 465 (< 0.1), 673 (0.66) 8.4 × 10 -4d
3
3
3 2
4
6
2n
2
1.7 × 10 -3b
a b
Data obtained with an uncertainty of 10 %; the relative luminescence quantum yields were measured at room temperature using [Ru(bpy) ]Cl 2
3
c -1 d
in degassed acetonitrile as a standard; vibronic-structured band with vibrational progressional spacings of ca. 1150-1320 cm ; the relative
luminescence quantum yields were measured at room temperature using quinine sulfate in 0.5 M H SO as a standard.
2
4
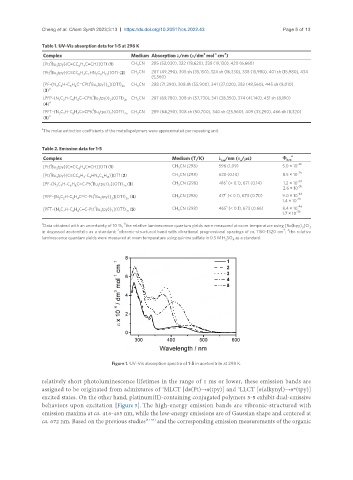
Figure 1. UV-Vis absorption spectra of 1-5 in acetonitrile at 298 K.
relatively short photoluminescence lifetimes in the range of 1 ms or lower, these emission bands are
3
3
assigned to be originated from admixtures of MLCT [dπ(Pt)→π(tpy)] and LLCT [π(alkynyl)→π*(tpy)]
excited states. On the other hand, platinum(II)-containing conjugated polymers 3-5 exhibit dual-emissive
behaviors upon excitation [Figure 5]. The high-energy emission bands are vibronic-structured with
emission maxima at ca. 416-465 nm, while the low-energy emissions are of Gaussian shape and centered at
ca. 672 nm. Based on the previous studies [51-54] and the corresponding emission measurements of the organic