Page 19 - Read Online
P. 19
Fabbrizi et al. Cancer Drug Resist 2020;3:775-90 I http://dx.doi.org/10.20517/cdr.2020.49 Page 781
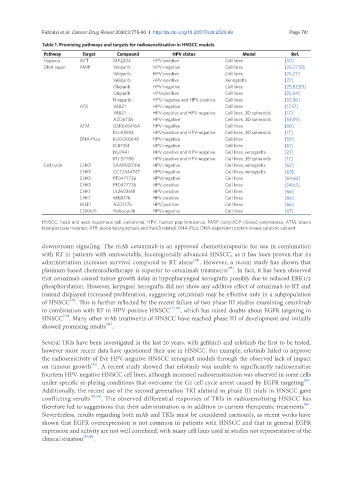
Table 1. Promising pathways and targets for radiosensitisation in HNSCC models
Pathway Target Compound HPV status Model Ref.
Hypoxia AKT MK2206 HPV-positive Cell lines [50]
DNA repair PARP Veliparib HPV-negative Cell lines [26,27,51]
Veliparib HPV-positive Cell lines [26,27]
Veliparib HPV-positive Xenografts [27]
Olaparib HPV-negative Cell lines [25,52,53]
Olaparib HPV-positive Cell lines [25,54]
Niraparib HPV-negative and HPV-positive Cell lines [55,56]
ATR VE821 HPV-negative Cell lines [17,57]
VE821 HPV-positive and HPV-negative Cell lines, 3D spheroids [17]
AZD6738 HPV-negative Cell lines, 3D spheroids [58,59]
ATM GSK645416A HPV-negative Cell lines [60]
KU-55933 HPV-positive and HPV-negative Cell lines, 3D spheroids [17]
DNA-Pkcs KU0060648 HPV-negative Cell lines [59]
IC87361 HPV-negative Cell lines [61]
NU7441 HPV-positive and HPV-negative Cell lines, xenografts [27]
KU-57788 HPV-positive and HPV-negative Cell lines, 3D spheroids [17]
Cell cycle CHK1 SAAR020106 HPV-negative Cell lines, xenografts [62]
CHK1 CCT2444747 HPV-negative Cell lines, xenografts [63]
CHK1 PF0477736 HPV-negative Cell lines [64,65]
CHK1 PF0477736 HPV-positive Cell lines [54,65]
CHK1 LY2603618 HPV-positive Cell lines [66]
CHK1 MK8776 HPV-positive Cell lines [66]
WEE1 AZD1775 HPV-positive Cell lines [66]
CDK4/6 Palbocyclib HPV-negative Cell lines [67]
HNSCC: head and neck squamous cell carcinoma; HPV: human papillomavirus; PARP: poly(ADP-ribose) polymerase; ATM: ataxia
telangiectasia mutated; ATR: ataxia telangiectasia and Rad-3 related; DNA-Pkcs: DNA-dependent protein kinase catalytic subunit
downstream signaling. The mAb cetuximab is an approved chemotherapeutic for use in combination
with RT in patients with unresectable, locoregionally advanced HNSCC, as it has been proven that its
[74]
administration increases survival compared to RT alone . However, a recent study has shown that
[75]
platinum-based chemoradiotherapy is superior to cetuximab treatments . In fact, it has been observed
that cetuximab caused tumor growth delay in hypopharyngeal xenografts possibly due to reduced ERK1/2
phosphorylation. However, laryngeal xenografts did not show any additive effect of cetuximab to RT and
instead displayed increased proliferation, suggesting cetuximab may be effective only in a subpopulation
of HNSCC . This is further reflected by the recent failure of two phase III studies examining cetuximab
[76]
in combination with RT in HPV-positive HNSCC [77,78] , which has raised doubts about EGFR targeting in
HNSCC . Many other mAb treatments of HNSCC have reached phase III of development and initially
[79]
[80]
showed promising results .
Several TKIs have been investigated in the last 20 years, with gefitinib and erlotinib the first to be tested,
however more recent data have questioned their use in HNSCC. For example, erlotinib failed to improve
the radiosensitivity of five HPV-negative HNSCC xenograft models through the observed lack of impact
on tumour growth . A recent study showed that erlotinib was unable to significantly radiosensitise
[81]
fourteen HPV-negative HNSCC cell lines, although increased radiosensitisation was observed in some cells
under specific re-plating conditions that overcame the G2 cell cycle arrest caused by EGFR targeting .
[82]
Additionally, the recent use of the second generation TKI afatinid in phase III trials in HNSCC gave
conflicting results [83,84] . The observed differential responses of TKIs in radiosensitising HNSCC has
[80]
therefore led to suggestions that their administration is in addition to current therapeutic treatments .
Nevertheless, results regarding both mAb and TKIs must be considered cautiously, as recent works have
shown that EGFR overexpression is not common in patients with HNSCC and that in general EGFR
expression and activity are not well correlated, with many cell lines used in studies not representative of the
clinical situation [85,86] .