Page 16 - Read Online
P. 16
Page 778 Fabbrizi et al. Cancer Drug Resist 2020;3:775-90 I http://dx.doi.org/10.20517/cdr.2020.49
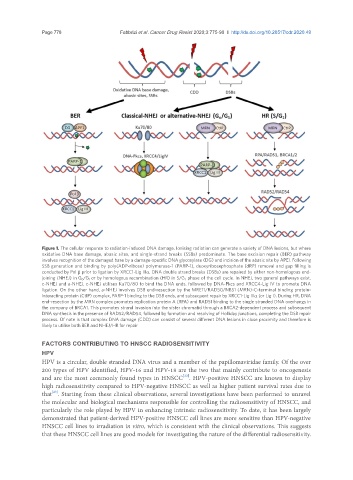
Figure 1. The cellular response to radiation-induced DNA damage. Ionising radiation can generate a variety of DNA lesions, but where
oxidative DNA base damage, abasic sites, and single-strand breaks (SSBs) predominate. The base excision repair (BER) pathway
involves recognition of the damaged base by a damage-specific DNA glycosylase (DG) and incision of the abasic site by APE1. Following
SSB generation and binding by poly(ADP-ribose) polymerase-1 (PARP-1), deoxyribosephosphate (dRP) removal and gap filling is
conducted by Pol b prior to ligation by XRCC1-Lig IIIa. DNA double strand breaks (DSBs) are repaired by either non-homologous end-
joining (NHEJ) in G 0 /G 1 or by homologous recombination (HR) in S/G 2 phase of the cell cycle. In NHEJ, two general pathways exist,
c-NHEJ and a-NHEJ. c-NHEJ utilises Ku70/80 to bind the DNA ends, followed by DNA-Pkcs and XRCC4-Lig IV to promote DNA
ligation. On the other hand, a-NHEJ involves DSB end-resection by the MRE11/RAD50/NBS1 (MRN)-C-terminal binding protein-
interacting protein (CtIP) complex, PARP-1 binding to the DSB ends, and subsequent repair by XRCC1-Lig IIIa (or Lig I). During HR, DNA
end-resection by the MRN complex promotes replication protein A (RPA) and RAD51 binding to the single stranded DNA overhangs in
the company of BRCA1. This promotes strand invasion into the sister chromatid through a BRCA2-dependent process and subsequent
DNA synthesis in the presence of RAD52/RAD54, followed by formation and resolving of Holliday junctions, completing the DSB repair
process. Of note is that complex DNA damage (CDD) can consist of several different DNA lesions in close proximity and therefore is
likely to utilise both BER and NHEJ/HR for repair
FACTORS CONTRIBUTING TO HNSCC RADIOSENSITIVITY
HPV
HPV is a circular, double stranded DNA virus and a member of the papillomaviridae family. Of the over
200 types of HPV identified, HPV-16 and HPV-18 are the two that mainly contribute to oncogenesis
[22]
and are the most commonly found types in HNSCC . HPV-positive HNSCC are known to display
high radiosensitivity compared to HPV-negative HNSCC as well as higher patient survival rates due to
[23]
that . Starting from these clinical observations, several investigations have been performed to unravel
the molecular and biological mechanisms responsible for controlling the radiosensitivity of HNSCC, and
particularly the role played by HPV in enhancing intrinsic radiosensitivity. To date, it has been largely
demonstrated that patient-derived HPV-positive HNSCC cell lines are more sensitive than HPV-negative
HNSCC cell lines to irradiation in vitro, which is consistent with the clinical observations. This suggests
that these HNSCC cell lines are good models for investigating the nature of the differential radiosensitivity.