Page 26 - Read Online
P. 26
Brettrager et al. Cancer Drug Resist 2019;2:1153-63 I http://dx.doi.org/10.20517/cdr.2019.91 Page 1159
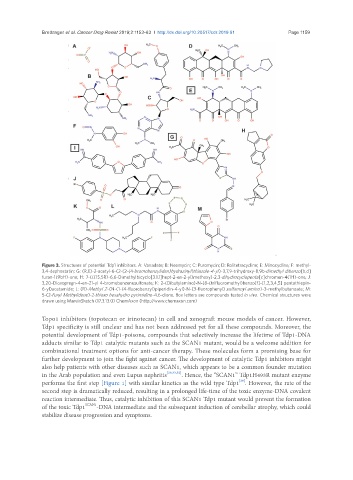
Figure 3. Structures of potential Tdp1 inhibitors. A: Vanadate; B: Neomycin; C: Puromycin; D: Rolitetracycline; E: Minocycline; F: methyl-
3,4-dephostatin; G: (R,E)-2-acetyl-6-(2-(2-(4-bromobenzyliden)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyl dibenzo[b,d]
furan-1(9bH)-one; H: 7-(((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methoxy)-2,3-dihydrocyclopenta[c]chromen-4(1H)-one; J:
3,20-Dioxopregn-4-en-21-yl 4-bromobenzenesulfonate; K: 2-(Dibutylamino)-N-(8-(trifluoromethyl)benzo[f]-[1,2,3,4,5] pentathiepin-
6-yl)acetamide; L: (R)-Methyl 2-(N-(1-(4-fluorobenzyl)piperidin-4-yl)-N-(3-fluorophenyl) sulfamoyl amino)-3-methylbutanoate; M:
5-(2-Furyl Methylidene)-2-thioxo hexahydro pyrimidine-4,6-dione. Box letters are compounds tested in vivo. Chemical structures were
drawn using MarvinSketch (17.3.13.0) ChemAxon (http://www.chemaxon.com)
Topo1 inhibitors (topotecan or irinotecan) in cell and xenograft mouse models of cancer. However,
Tdp1 specificity is still unclear and has not been addressed yet for all these compounds. Moreover, the
potential development of Tdp1-poisons, compounds that selectively increase the lifetime of Tdp1-DNA
adducts similar to Tdp1 catalytic mutants such as the SCAN1 mutant, would be a welcome addition for
combinational treatment options for anti-cancer therapy. These molecules form a promising base for
further development to join the fight against cancer. The development of catalytic Tdp1 inhibitors might
also help patients with other diseases such as SCAN1, which appears to be a common founder mutation
in the Arab population and even Lupus nephritis [29,33,52] . Hence, the “SCAN1” Tdp1H493R mutant enzyme
[26]
performs the first step [Figure 1] with similar kinetics as the wild type Tdp1 . However, the rate of the
second step is dramatically reduced, resulting in a prolonged life-time of the toxic enzyme-DNA covalent
reaction intermediate. Thus, catalytic inhibition of this SCAN1 Tdp1 mutant would prevent the formation
of the toxic Tdp1 SCAN1 -DNA intermediate and the subsequent induction of cerebellar atrophy, which could
stabilize disease progression and symptoms.