Page 26 - Read Online
P. 26
Page 346 Seno et al. Cancer Drug Resist 2019;2:335-50 I http://dx.doi.org/10.20517/cdr.2019.01
A B
C D
F
E
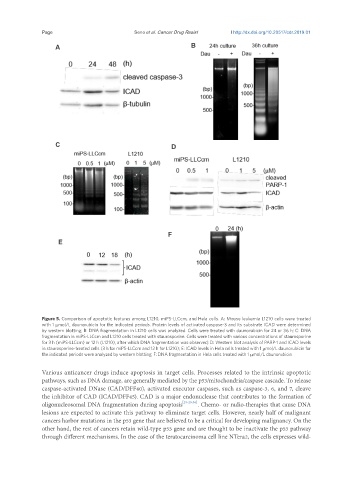
Figure 5. Comparison of apoptotic features among L1210, miPS-LLCcm, and Hela cells. A: Mouse leukemia L1210 cells were treated
with 1 μmol/L daunorubicin for the indicated periods. Protein levels of activated caspase-3 and its substrate ICAD were determined
by western blotting; B: DNA fragmentation in L1210 cells was analyzed. Cells were treated with daunorubicin for 24 or 36 h; C: DNA
fragmentation in miPS-LLCcm and L1210 cells treated with staurosporine. Cells were treated with various concentrations of staurosporine
for 3 h (miPS-LLCcm) or 12 h (L1210), after which DNA fragmentation was observed; D: Western blot analysis of PARP-1 and ICAD levels
in staurosporine-treated cells (3 h for miPS-LLCcm and 12 h for L1210); E: ICAD levels in Hela cells treated with 1 μmol/L daunorubicin for
the indicated periods were analyzed by western blotting; F: DNA fragmentation in Hela cells treated with 1 μmol/L daunorubicin
Various anticancer drugs induce apoptosis in target cells. Processes related to the intrinsic apoptotic
pathways, such as DNA damage, are generally mediated by the p53/mitochondria/caspase cascade. To release
caspase-activated DNase (CAD/DFF40), activated executor caspases, such as caspase-3, 6, and 7, cleave
the inhibitor of CAD (ICAD/DFF45). CAD is a major endonuclease that contributes to the formation of
oligonucleosomal DNA fragmentation during apoptosis [23-25,36] . Chemo- or radio-therapies that cause DNA
lesions are expected to activate this pathway to eliminate target cells. However, nearly half of malignant
cancers harbor mutations in the p53 gene that are believed to be a critical for developing malignancy. On the
other hand, the rest of cancers retain wild-type p53 gene and are thought to be inactivate the p53 pathway
through different mechanisms. In the case of the teratocarcinoma cell line NTera2, the cells expresses wild-