Page 77 - Read Online
P. 77
De Mattia et al. Cancer Drug Resist 2019;2:116-30 I http://dx.doi.org/10.20517/cdr.2019.04 Page 119
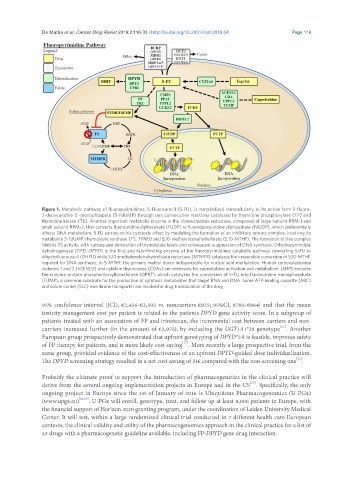
Figure 1. Metabolic pathway of fluoropyrimidines. 5-Fluorouracil (5-FU), is metabolized intracellularly to its active form 5-fluoro-
2-deoxyuridine-5’-monophospate (5-FdUMP) through two consecutive reactions catalyzed by thymidine phosphorylase (TP) and
thymidine kinase (TK). Another important metabolic enzyme is the ribonucleotide reductase, composed of large subunit RRM-1 and
small subunit RRM-2, that converts fluorouridine diphosphate (FUDP) to fluorodeoxyuridine diphosphate (FdUDP), which preferentially
affects DNA metabolism. 5-FU carries on his cytotoxic effect by mediating the formation of an inhibitory ternary complex, involving its
metabolite 5-FdUMP, thymidylate synthase (TS, TYMS) and 5,10-methylentetrahydrofolate (5,10-MTHF). The formation of this complex
inhibits TS activity, with subsequent diminution of thymidylate levels and consequent suppression of DNA synthesis. Dihydropyrimidine
dehydrogenase (DPD, DPYD) is the first and rate-limiting enzyme of the fluoropyrimidines catabolic pathway converting 5-FU to
dihydrofluorouracil (DHFU) while 5,10-methylenetetrahydrofolate reductase (MTHFR) catalyzes the irreversible conversion of 5,10-MTHF,
required for DNA synthesis, to 5-MTHF, the primary methyl donor indispensable for nucleic acid methylation. Human carboxylesterase
isoforms 1 and 2 (hCES1/2) and cytidine deaminases (CDAs) are necessary for capecitabine activation and metabolism. UMPS encodes
the enzyme orotate phosphoribosyltransferase (OPRT), which catalyzes the conversion of 5-FU into fluorouridine monophosphate
(FUMP), a common substrate for the production of cytotoxic metabolites that target RNA and DNA. Some ATP-binding cassette (ABC)
and solute carrier (SLC) membrane transporter are involved in drug translocation of the drug
95% confidence interval [(CI), €2,456-€3,505 vs. noncarriers €825; 95%CI, €785-€864] and that the mean
toxicity management cost per patient is related to the patients DPYD gene activity score. In a subgroup of
patients treated with an association of FP and irinotecan, the incremental cost between carriers and non-
[25]
carriers increased further (in the amount of €2,975), by including the UGT1A1*28 genotype . Another
European group prospectively demonstrated that upfront genotyping of DPYD*2A is feasible, improves safety
[17]
of FP therapy for patients, and is more likely cost saving . More recently a large prospective trial, from the
same group, provided evidence of the cost-effectiveness of an upfront DPYD-guided dose individualization.
[26]
The DPYD screening strategy resulted in a net cost saving of 51€ compared with the non-screening one .
Probably the ultimate proof to support the introduction of pharmacogenetics in the clinical practice will
[27]
derive from the several ongoing implementation projects in Europe and in the US . Specifically, the only
ongoing project in Europe since the 1st of January of 2016 is Ubiquitous Pharmacogenomics (U-PGx)
(www.upgx.eu) [28,29] . U-PGx will enroll, genotype, treat, and follow up at least 8,000 patients in Europe, with
the financial support of Horizon 2020 granting program, under the coordination of Leiden University Medical
Center. It will test, within a large randomized clinical trial conducted in 7 different health care European
contexts, the clinical validity and utility of the pharmacogenomics approach in the clinical practice for a list of
43 drugs with a pharmacogenetic guideline available, including FP-DPYD gene drug interaction.