Page 134 - Read Online
P. 134
Page 668 Murthy et al. Cancer Drug Resist 2019;2:665-79 I http://dx.doi.org/10.20517/cdr.2019.002
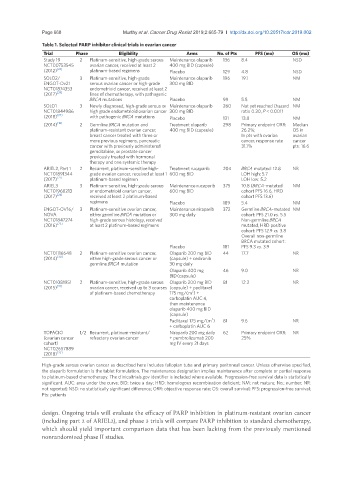
Table 1. Selected PARP inhibitor clinical trials in ovarian cancer
Trial Phase Eligibility Arms No. of Pts PFS (mo) OS (mo)
Study 19 2 Platinum-sensitive, high-grade serous Maintenance olaparib 136 8.4 NSD
NCT00753545 ovarian cancer, received at least 2 400 mg BID (capsule)
(2012) [22] platinum-based regimens Placebo 129 4.8 NSD
SOLO2/ 3 Platinum-sensitive, high-grade Maintenance olaparib 196 19.1 NM
ENGOT-Ov21 serous ovarian cancer or high-grade 300 mg BID
NCT01874353 endometrioid cancer, received at least 2
(2017) [29] lines of chemotherapy, with pathogenic
BRCA mutations Placebo 99 5.5 NM
SOLO1 3 Newly diagnosed, high-grade serous or Maintenance olaparib 260 Not yet reached (hazard NM
NCT01844986 high grade endometrioid ovarian cancer 300 mg BID ratio 0.30, P < 0.001)
(2018) [27] with pathogenic BRCA mutations Placebo 131 13.8 NM
(2014) [18] 2 Germline BRCA mutation and Treatment olaparib 298 Primary endpoint ORR: Median
platinum-resistant ovarian cancer, 400 mg BID (capsule) 26.2%; OS in
breast cancer treated with three or In pts with ovarian ovarian
more previous regimens, pancreatic cancer, response rate cancer
cancer with previously administered 31.1% pts: 16.6
gemcitabine, or prostate cancer
previously treated with hormonal
therapy and one systemic therapy
ARIEL2, Part 1 2 Recurrent, platinum-sensitive high- Treatment rucaparib 204 BRCA mutated: 12.8 NR
NCT01891344 grade ovarian cancer, received at least 1 600 mg BID LOH high: 5.7
(2017) [21] platinum-based regimen LOH low: 5.2
ARIEL3 3 Platinum-sensitive, high-grade serous Maintenance rucaparib 375 10.8 (BRCA-mutated NM
NCT01968213 or endometrioid ovarian cancer, 600 mg BID cohort PFS 16.6, HRD
(2017) [24] received at least 2 platinum-based cohort PFS 13.6)
regimens Placebo 189 5.4 NM
ENGOT-OV16/ 3 Platinum-sensitive ovarian cancer, Maintenance niraparib 372 Germline BRCA-mutated NM
NOVA either germline BRCA mutation or 300 mg daily cohort: PFS 21.0 vs. 5.5
NCT01847274 high-grade serous histology, received Non-germline BRCA
(2016) [23] at least 2 platinum-based regimens mutated, HRD positive
cohort: PFS 12.9 vs. 3.8
Overall non-germline
BRCA mutated cohort:
Placebo 181 PFS 9.3 vs. 3.9
NCT01116648 2 Platinum-sensitive ovarian cancer, Olaparib 200 mg BID 44 17.7 NR
(2014) [30] either high-grade serous cancer or (capsule) + cediranib
germline BRCA mutation 30 mg daily
Olaparib 400 mg 46 9.0 NR
BID (capsule)
NCT01081951 2 Platinum-sensitive, high-grade serous Olaparib 200 mg BID 81 12.2 NR
(2015) [31] ovarian cancer, received up to 3 courses (capsule) + paclitaxel
2
of platinum-based chemotherapy 175 mg/(m ) +
carboplatin AUC 4,
then maintenance
olaparib 400 mg BID
(capsule)
2
Paclitaxel 175 mg/(m ) 81 9.6 NR
+ carboplatin AUC 6
TOPACIO 1/2 Recurrent, platinum-resistant/ Niraparib 200 mg daily 62 Primary endpoint ORR: NR
(ovarian cancer refractory ovarian cancer + pembrolizumab 200 25%
cohort) mg IV every 21 days
NCT02657889
(2018) [32]
High-grade serous ovarian cancer as described here includes fallopian tube and primary peritoneal cancer. Unless otherwise specified,
the olaparib formulation is the tablet formulation. The maintenance designation implies maintenance after complete or partial response
to platinum-based chemotherapy. The clinicaltrials.gov identifier is included where available. Progression-free survival data is statistically
significant. AUC: area under the curve; BID: twice a day; HRD: homologous recombination deficient; NM: not mature; No.: number; NR:
not reported; NSD: no statistically significant difference; ORR: objective response rate; OS: overall survival; PFS: progression-free survival;
Pts: patients
design. Ongoing trials will evaluate the efficacy of PARP inhibition in platinum-resistant ovarian cancer
(including part 2 of ARIEL2), and phase 3 trials will compare PARP inhibition to standard chemotherapy,
which should yield important comparison data that has been lacking from the previously mentioned
nonrandomized phase II studies.