Page 133 - Read Online
P. 133
Murthy et al. Cancer Drug Resist 2019;2:665-79 I http://dx.doi.org/10.20517/cdr.2019.002 Page 667
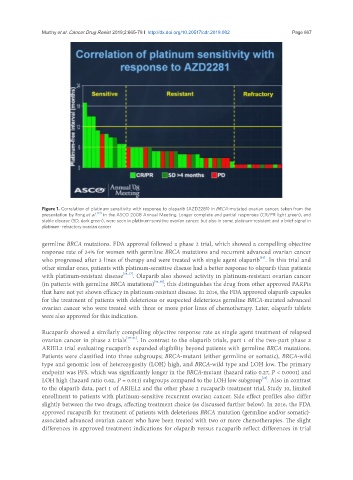
Figure 1. Correlation of platinum sensitivity with response to olaparib (AZD2281) in BRCA-mutated ovarian cancer, taken from the
presentation by Fong et al. [14] in the ASCO 2008 Annual Meeting. Longer complete and partial responses (CR/PR light green), and
stable disease (SD, dark green), were seen in platinum-sensitive ovarian cancer, but also in some platinum-resistant and a brief signal in
platinum- refractory ovarian cancer
germline BRCA mutations. FDA approval followed a phase 2 trial, which showed a compelling objective
response rate of 34% for women with germline BRCA mutations and recurrent advanced ovarian cancer
[16]
who progressed after 3 lines of therapy and were treated with single agent olaparib . In this trial and
other similar ones, patients with platinum-sensitive disease had a better response to olaparib than patients
with platinum-resistant disease [14,17] . Olaparib also showed activity in platinum-resistant ovarian cancer
(in patients with germline BRCA mutations) [14,18] ; this distinguishes the drug from other approved PARPis
that have not yet shown efficacy in platinum-resistant disease. In 2014, the FDA approved olaparib capsules
for the treatment of patients with deleterious or suspected deleterious germline BRCA-mutated advanced
ovarian cancer who were treated with three or more prior lines of chemotherapy. Later, olaparib tablets
were also approved for this indication.
Rucaparib showed a similarly compelling objective response rate as single agent treatment of relapsed
ovarian cancer in phase 2 trials [19-21] . In contrast to the olaparib trials, part 1 of the two-part phase 2
ARIEL2 trial evaluating rucaparib expanded eligibility beyond patients with germline BRCA mutations.
Patients were classified into three subgroups; BRCA-mutant (either germline or somatic), BRCA-wild
type and genomic loss of heterozygosity (LOH) high, and BRCA-wild type and LOH low. The primary
endpoint was PFS, which was significantly longer in the BRCA-mutant (hazard ratio 0.27, P < 0.0001) and
[21]
LOH high (hazard ratio 0.62, P = 0.011) subgroups compared to the LOH low subgroup . Also in contrast
to the olaparib data, part 1 of ARIEL2 and the other phase 2 rucaparib treatment trial, Study 10, limited
enrollment to patients with platinum-sensitive recurrent ovarian cancer. Side effect profiles also differ
slightly between the two drugs, affecting treatment choice (as discussed further below). In 2016, the FDA
approved rucaparib for treatment of patients with deleterious BRCA mutation (germline and/or somatic)-
associated advanced ovarian cancer who have been treated with two or more chemotherapies. The slight
differences in approved treatment indications for olaparib versus rucaparib reflect differences in trial