Page 120 - Read Online
P. 120
Shek et al. Cancer Drug Resist 2019;2:69-81 I http://dx.doi.org/10.20517/cdr.2018.20 Page 71
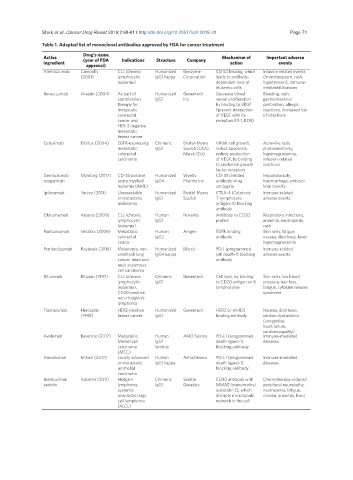
Table 1. Adapted list of monoclonal antibodies approved by FDA for cancer treatment
Drug’s name
Active (year of FDA Indications Structure Company Mechanism of Important adverse
ingredient action events
approval)
Alemtuzumab Campath CLL (chronic Humanized Genzyme CD 52 binding, which Infusion-related events
(2001) lymphocytic IgG1 kappa Corporation leads to antibody- (bronchospasm, rash,
leukemia) dependent lysis of hypotension), immune-
leukemic cells mediated diseases
Bevacizumab Avastin (2004) As part of Humanized Genentech Decrease blood Bleeding, rash,
combination IgG1 Inc. vessel proliferation gastrointestinal
therapy for by binding to VEGF perforation, allergic
metastatic (prevent interaction reactions, increased risk
colorectal of VEGF with its of infections
cancer and receptors Flt-1, KDR)
HER-2 negative
metastatic
breast cancer
Cetuximab Erbitux (2004) EGFR-expressing Chimeric Bristol-Myers Inhibit cell growth, Acne-like rash,
metastatic IgG1 Squibb (USA), induct apoptosis, photosensitivity,
colorectal Merck (EU) reduce production hypomagnesemia,
carcinoma of VEGF, by binding infusion-related
to epidermal growth reactions
factor receptors
Gemtuzumab Mylotarg (2017) CD-33 positive Humanized Wyeth CD-33 directed Hepatotoxicity,
ozogamicin acute myeloid IgG4 Pharms Inc. antibody-drug haemorrhage, embryo-
leukemia (AML) conjugate fetal toxicity
Ipilimumab Yervoy (2011) Unresectable Humanized Bristol-Myers CTLA-4 (Cytotoxic Immune-related
or metastatic IgG1 Squibb T-lymphocyte adverse events
melanoma antigen-4) blocking
antibody
Ofatumumab Arzerra (2009) CLL (chronic Human Novartis Antibody to CD20 Respiratory infections,
lymphocytic IgG1 protein anaemia, neutropenia,
leukemia) rash
Panitumumab Vectibix (2006) Metastatic Human Amgen EGFR binding Skin rash, fatigue,
colorectal IgG2 antibody nausea, diarrhoea, fever,
cancer hypomagnesemia
Pembrolizumab Keytruda (2014) Melanoma, non- Humanized Merck PD-1 (programmed Immune-related
small cell lung IgG4 kappa cell death-1) blocking adverse events
cancer, head and antibody
neck squamous
cell carcinoma
Rituximab Rituxan (1997) CLL (chronic Chimeric Genentech Cell lysis, by binding Skin rash, low blood
lymphocytic IgG1 to CD20 antigen on B pressure, hair loss,
leukemia), lymphocytes fatigue, cytokine release
CD20-positive syndrome
non-Hodgkin’s
lymphoma
Trastuzumab Herceptin HER2-positive Humanized Genentech HER2 (c-erb82) Nausea, diarrhoea,
(1998) breast cancer IgG1 binding antibody cardiac dysfunction
(congestive
heart failure,
cardiomyopathy)
Avelumab Bavencio (2017) Metastatic Human AMD Serono PD-L1 (programmed Immune-mediated
Merkel cell IgG1 death ligand-1) diseases
carcinoma lambda blocking antibody
(MCC)
Durvalumab Imfinzi (2017) Locally advanced Human AstraZeneca PD-L1 (programmed Immune-mediated
or metastatic IgG1 kappa death ligand-1) diseases
urothelial blocking antibody
carcinoma
Brentuximab Adcetris (2011) Hodgkin Chimeric Seattle CD30 antibody with Chemotherapy-induced
vedotin lymphoma, IgG1 Genetics MMAE (monomethyl peripheral neuropathy,
systemic auristatin E), which neutropenia, fatigue,
anaplastic large disrupts microtubule nausea, anaemia, fever
cell lymphoma network in the cell
(ALCL)