Page 9 - Read Online
P. 9
Giovannetti et al. Cancer Drug Resist 2018;1:82-6 I http://dx.doi.org/10.20517/cdr.2018.05 Page 84
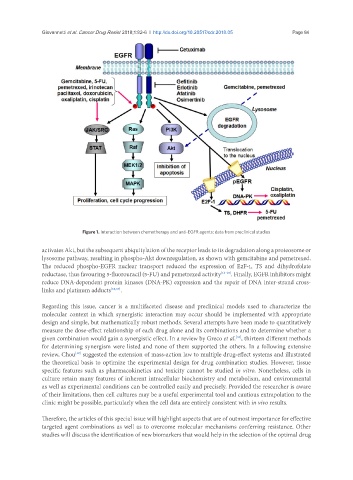
Figure 1. Interaction between chemotherapy and anti-EGFR agents: data from preclinical studies
activates Akt, but the subsequent ubiquitylation of the receptor leads to its degradation along a proteosome or
lysosome pathway, resulting in phospho-Akt downregulation, as shown with gemcitabine and pemetrexed.
The reduced phospho-EGFR nuclear transport reduced the expression of E2F-1, TS and dihydrofolate
reductase, thus favouring 5-fluorouracil (5-FU) and pemetrexed activity [13-15] . Finally, EGFR inhibitors might
reduce DNA-dependent protein kinases (DNA-PK) expression and the repair of DNA inter-strand cross-
links and platinum adducts [16,17] .
Regarding this issue, cancer is a multifaceted disease and preclinical models used to characterize the
molecular context in which synergistic interaction may occur should be implemented with appropriate
design and simple, but mathematically robust methods. Several attempts have been made to quantitatively
measure the dose-effect relationship of each drug alone and its combinations and to determine whether a
given combination would gain a synergistic effect. In a review by Greco et al. , thirteen different methods
[18]
for determining synergism were listed and none of them supported the others. In a following extensive
review, Chou suggested the extension of mass-action law to multiple drug-effect systems and illustrated
[19]
the theoretical basis to optimize the experimental design for drug combination studies. However, tissue
specific features such as pharmacokinetics and toxicity cannot be studied in vitro. Nonetheless, cells in
culture retain many features of inherent intracellular biochemistry and metabolism, and environmental
as well as experimental conditions can be controlled easily and precisely. Provided the researcher is aware
of their limitations, then cell cultures may be a useful experimental tool and cautious extrapolation to the
clinic might be possible, particularly when the cell data are entirely consistent with in vivo results.
Therefore, the articles of this special issue will highlight aspects that are of outmost importance for effective
targeted agent combinations as well as to overcome molecular mechanisms conferring resistance. Other
studies will discuss the identification of new biomarkers that would help in the selection of the optimal drug