Page 14 - Read Online
P. 14
Page 206 Wu et al. Cancer Drug Resist 2018;1:204-18 I http://dx.doi.org/10.20517/cdr.2018.16
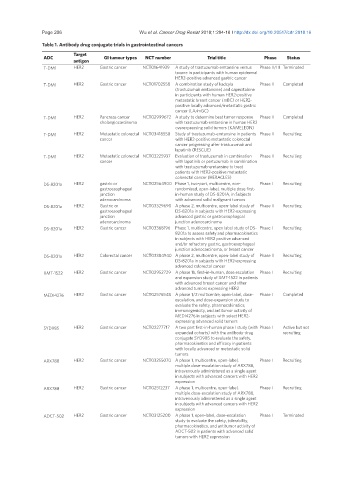
Table 1. Antibody drug conjugate trials in gastrointestinal cancers
Target
ADC GI tumour types NCT number Trial title Phase Status
antigen
T-DM1 HER2 Gastric cancer NCT01641939 A study of trastuzumab emtansine versus Phase II/III Terminated
taxane in participants with human epidermal
HER2-positive advanced gastric cancer
T-DM1 HER2 Gastric cancer NCT01702558 A combination study of kadcyla Phase II Completed
(trastuzumab emtansine) and capecitabine
in participants with human HER2-positive
metastatic breast cancer (mBC) or HER2-
positive locally advanced/metastatic gastric
cancer (LA/mGC)
T-DM1 HER2 Pancreas cancer NCT02999672 A study to determine best tumor response Phase II Completed
cholangiocarcinoma with trastuzumab emtansine in human HER2
overexpressing solid tumors (KAMELEON)
T-DM1 HER2 Metastatic colorectal NCT03418558 Study of trastuzumab-emtansine in patients Phase II Recruiting
cancer with HER2-positive metastatic colorectal
cancer progressing after trastuzumab and
lapatinib (RESCUE)
T-DM1 HER2 Metastatic colorectal NCT03225937 Evaluation of trastuzumab in combination Phase II Recruiting
cancer with lapatinib or pertuzumab in combination
with trastuzumab-emtansine to treat
patients with HER2-positive metastatic
colorectal cancer (HERACLES)
DS-8201a HER2 gastric or NCT02564900 Phase 1, two-part, multicentre, non- Phase I Recruiting
gastroesophageal randomized, open-label, multiple dose first-
junction in-human study of DS-8201A, in Subjects
adenocarcinoma with advanced solid malignant tumors
DS-8201a HER2 Gastric or NCT03329690 A phase 2, multicentre, open-label study of Phase II Recruiting
gastroesophageal DS-8201a in subjects with HER2-expressing
junction advanced gastric or gastroesophageal
adenocarcinoma junction adenocarcinoma
DS-8201a HER2 Gastric cancer NCT03368196 Phase 1, multicentre, open label study of DS- Phase I Recruiting
8201a to assess safety and pharmacokinetics
in subjects with HER2 positive advanced
and/or refractory gastric, gastroesophageal
junction adenocarcinoma, or breast cancer
DS-8201a HER2 Colorectal cancer NCT03384940 A phase 2, multicentre, open-label study of Phase II Recruiting
DS-8201a in subjects with HER2-expressing
advanced colorectal cancer
XMT-1522 HER2 Gastric cancer NCT02952729 A phase 1b, first-in-human, dose escalation Phase I Recruiting
and expansion study of XMT-1522 in patients
with advanced breast cancer and other
advanced tumors expressing HER2
MEDI4276 HER2 Gastric cancer NCT02576548 A phase 1/2 multicentre, open-label, dose- Phase I Completed
escalation, and dose-expansion study to
evaluate the safety, pharmacokinetics,
immunogenicity, and antitumor activity of
MEDI4276 in subjects with select HER2-
expressing advanced solid tumors
SYD985 HER2 Gastric cancer NCT02277717 A two part first-in-human phase I study (with Phase I Active but not
expanded cohorts) with the antibody-drug recruiting
conjugate SYD985 to evaluate the safety,
pharmacokinetics and efficacy in patients
with locally advanced or metastatic solid
tumors
ARX788 HER2 Gastric cancer NCT03255070 A phase 1, multicentre, open-label, Phase I Recruiting
multiple dose-escalation study of ARX788,
intravenously administered as a single agent
in subjects with advanced cancers with HER2
expression
ARX788 HER2 Gastric cancer NCT02512237 A phase 1, multicentre, open-label, Phase I Recruiting
multiple dose-escalation study of ARX788,
intravenously administered as a single agent
in subjects with advanced cancers with HER2
expression
ADCT-502 HER2 Gastric cancer NCT03125200 A phase 1, open-label, dose-escalation Phase I Terminated
study to evaluate the safety, tolerability,
pharmacokinetics, and antitumor activity of
ADCT-502 in patients with advanced solid
tumors with HER2 expression