Page 15 - Read Online
P. 15
Wu et al. Cancer Drug Resist 2018;1:204-18 I http://dx.doi.org/10.20517/cdr.2018.16 Page 207
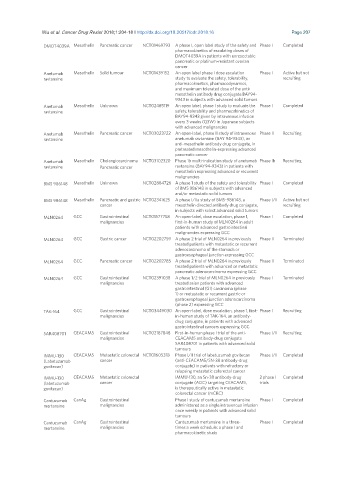
DMOT4039A Mesothelin Pancreatic cancer NCT01469793 A phase I, open label study of the safety and Phase I Completed
pharmacokinetics of escalating doses of
DMOT4039A in patients with unresectable
pancreatic or platinum-resistant ovarian
cancer
Anetumab Mesothelin Solid tumour NCT01439152 An open label phase I dose escalation Phase I Active but not
ravtansine study to evaluate the safety, tolerability, recruiting
pharmacokinetics, pharmacodynamics,
and maximum tolerated dose of the anti-
mesothelin antibody drug conjugate BAY94-
9343 in subjects with advanced solid tumors
Anetumab Mesothelin Unknown NCT02485119 An open label, phase I study to evaluate the Phase I Completed
ravtansine safety, tolerability and pharmacokinetics of
BAY94-9343 given by intravenous infusion
every 3 weeks (Q3W) in Japanese subjects
with advanced malignancies
Anetumab Mesothelin Pancreatic cancer NCT03023722 An open-label, phase II study of intravenous Phase II Recruiting
ravtansine anetumab ravtansine (BAY 94-9343), an
anti-mesothelin antibody drug conjugate, in
pretreated mesothelin-expressing advanced
pancreatic cancer
Anetumab Mesothelin Cholangiocarcinoma NCT03102320 Phase 1b multi-indication study of anetumab Phase Ib Recruiting
ravtansine Pancreatic cancer ravtansine (BAY94-9343) in patients with
mesothelin expressing advanced or recurrent
malignancies
BMS 986148 Mesothelin Unknown NCT02884726 A phase 1 study of the safety and tolerability Phase I Completed
of BMS 986148 in subjects with advanced
and/or metastatic solid tumors
BMS 986148 Mesothelin Pancreatic and gastric NCT02341625 A phase I/IIa study of BMS-986148, a Phase I/II Active but not
cancer mesothelin directed antibody drug conjugate, recruiting
in subjects with select advanced solid tumors
MLN0264 GCC Gastrointestinal NCT01577758 An open-label, dose escalation, phase 1, Phase I Completed
malignancies first-in-human study of MLN0264 in adult
patients with advanced gastrointestinal
malignancies expressing GCC
MLN0264 GCC Gastric cancer NCT02202759 A phase 2 trial of MLN0264 in previously Phase II Terminated
treated patients with metastatic or recurrent
adenocarcinoma of the stomach or
gastroesophageal junction expressing GCC
MLN0264 GCC Pancreatic cancer NCT02202785 A phase 2 trial of MLN0264 in previously Phase II Terminated
treated patients with advanced or metastatic
pancreatic adenocarcinoma expressing GCC
MLN0264 GCC Gastrointestinal NCT02391038 A phase 1/2 trial of MLN0264 in previously Phase I Terminated
malignancies treated asian patients with advanced
gastrointestinal (GI) carcinoma (phase
1) or metastatic or recurrent gastric or
gastroesophageal junction adenocarcinoma
(phase 2) expressing GCC
TAK-164 GCC Gastrointestinal NCT03449030 An open-label, dose escalation, phase 1, first- Phase I Recruiting
malignancies in-human study of TAK-164, an antibody-
drug conjugate, in patients with advanced
gastrointestinal cancers expressing GCC
SAR408701 CEACAM5 Gastrointestinal NCT02187848 First-in-human phase I trial of the anti- Phase I/II Recruiting
malignancies CEACAM5 antibody-drug conjugate
SAR408701 in patients with advanced solid
tumours
IMMU-130 CEACAM5 Metastatic colorectal NCT01605318 Phase I/II trial of labetuzumab govitecan Phase I/II Completed
(Labetuzumab cancer (anti-CEACAM5/SN-38 antibody-drug
govitecan) conjugate) in patients with refractory or
relapsing metastatic colorectal cancer
IMMU-130 CEACAM5 Metastatic colorectal IMMU-130, an Sn-38 antibody-drug 2 phase I Completed
(labetuzumab cancer conjugate (ADC) targeting CEACAM5, trials
govitecan) is therapeutically active in metastatic
colorectal cancer (mCRC)
Cantuzumab CanAg Gastrointestinal Phase I study of cantuzumab mertansine Phase I Completed
mertansine malignancies administered as a single intravenous infusion
once weekly in patients with advanced solid
tumours
Cantuzumab CanAg Gastrointestinal Cantuzumab mertansine in a three- Phase I Completed
mertansine malignancies times a week schedule: a phase I and
pharmacokinetic study