Page 88 - Read Online
P. 88
Page 236 Van Der Steen et al. Cancer Drug Resist 2018;1:230-49 I http://dx.doi.org/10.20517/cdr.2018.13
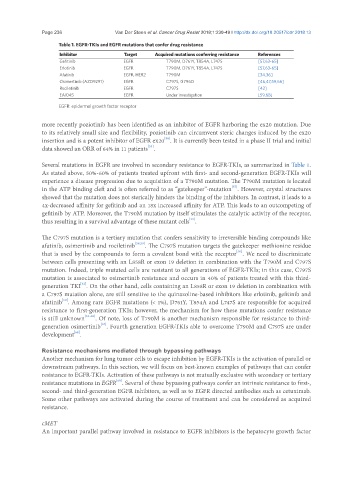
Table 1. EGFR-TKIs and EGFR mutations that confer drug resistance
Inhibitor Target Acquired mutations conferring resistance References
Gefitinib EGFR T790M, D761Y, T854A, L747S [57,63-65]
Erlotinib EGFR T790M, D761Y, T854A, L747S [57,63-65]
Afatinib EGFR, HER2 T790M [34,36]
Osimertinib (AZD9291) EGFR C797S, G796D [46,47,59,66]
Rociletinib EGFR C797S [42]
EAI045 EGFR Under investigation [59,68]
EGFR: epidermal growth factor receptor
more recently poziotinib has been identified as an inhibitor of EGFR harboring the ex20 mutation. Due
to its relatively small size and flexibility, poziotinib can circumvent steric changes induced by the ex20
[56]
insertion and is a potent inhibitor of EGFR ex20 . It is currently been tested in a phase II trial and initial
[55]
data showed an ORR of 64% in 11 patients .
Several mutations in EGFR are involved in secondary resistance to EGFR-TKIs, as summarized in Table 1.
As stated above, 50%-60% of patients treated upfront with first- and second-generation EGFR-TKIs will
experience a disease progression due to acquisition of a T790M mutation. The T790M mutation is located
[57]
in the ATP binding cleft and is often referred to as “gatekeeper”-mutation . However, crystal structures
showed that the mutation does not sterically hinders the binding of the inhibitors. In contrast, it leads to a
4x-decreased affinity for gefitinib and an 18x increased affinity for ATP. This leads to an outcompeting of
gefitinib by ATP. Moreover, the T790M mutation by itself stimulates the catalytic activity of the receptor,
[39]
thus resulting in a survival advantage of these mutant cells .
The C797S mutation is a tertiary mutation that confers sensitivity to irreversible binding compounds like
afatinib, osimertinib and rociletinib [58,59] . The C797S mutation targets the gatekeeper methionine residue
that is used by the compounds to form a covalent bond with the receptor . We need to discriminate
[60]
between cells presenting with an L858R or exon 19 deletion in combination with the T790M and C797S
mutation. Indeed, triple mutated cells are resistant to all generations of EGFR-TKIs; in this case, C797S
mutation is associated to osimertinib resistance and occurs in 40% of patients treated with this third-
[61]
generation TKI . On the other hand, cells containing an L858R or exon 19 deletion in combination with
a C797S mutation alone, are still sensitive to the quinazoline-based inhibitors like erlotinib, gefitinib and
[62]
afatinib . Among rare EGFR mutations (< 1%), D761Y, T854A and L747S are responsible for acquired
resistance to first-generation TKIs; however, the mechanism for how these mutations confer resistance
is still unknown [63-66] . Of note, loss of T790M is another mechanism responsible for resistance to third-
[67]
generation osimertinib . Fourth generation EGFR-TKIs able to overcome T790M and C797S are under
[68]
development .
Resistance mechanisms mediated through bypassing pathways
Another mechanism for lung tumor cells to escape inhibition by EGFR-TKIs is the activation of parallel or
downstream pathways. In this section, we will focus on best-known examples of pathways that can confer
resistance to EGFR-TKIs. Activation of these pathways is not mutually exclusive with secondary or tertiary
[69]
resistance mutations in EGFR . Several of these bypassing pathways confer an intrinsic resistance to first-,
second- and third-generation EGFR inhibitors, as well as to EGFR directed antibodies such as cetuximab.
Some other pathways are activated during the course of treatment and can be considered as acquired
resistance.
cMET
An important parallel pathway involved in resistance to EGFR inhibitors is the hepatocyte growth factor