Page 44 - Read Online
P. 44
Sendino et al. Cancer Drug Resist 2018;1:139-63 I http://dx.doi.org/10.20517/cdr.2018.09 Page 143
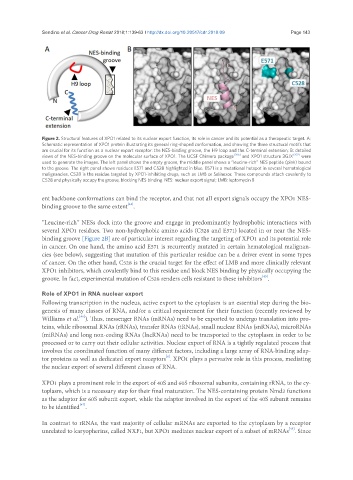
Figure 2. Structural features of XPO1 related to its nuclear export function, its role in cancer and its potential as a therapeutic target. A:
Schematic representation of XPO1 protein illustrating its general ring-shaped conformation, and showing the three structural motifs that
are crucial for its function as a nuclear export receptor: the NES-binding groove, the H9 loop and the C-terminal extension; B: detailed
views of the NES-binding groove on the molecular surface of XPO1. The UCSF Chimera package [206] and XPO1 structure 3GJX [207] were
used to generate the images. The left panel shows the empty groove, the middle panel shows a “leucine-rich” NES peptide (pink) bound
to the groove. The right panel shows residues E571 and C528 highlighted in blue. E571 is a mutational hotspot in several hematological
malignancies. C528 is the residue targeted by XPO1-inhibiting drugs, such as LMB or Selinexor. These compounds attach covalently to
C528 and physically occupy the groove, blocking NES binding. NES: nuclear export signal; LMB: leptomycin B
ent backbone conformations can bind the receptor, and that not all export signals occupy the XPO1 NES-
[44]
binding groove to the same extent .
“Leucine-rich” NESs dock into the groove and engage in predominantly hydrophobic interactions with
several XPO1 residues. Two non-hydrophobic amino acids (C528 and E571) located in or near the NES-
binding groove [Figure 2B] are of particular interest regarding the targeting of XPO1 and its potential role
in cancer. On one hand, the amino acid E571 is recurrently mutated in certain hematological malignan-
cies (see below), suggesting that mutation of this particular residue can be a driver event in some types
of cancer. On the other hand, C528 is the crucial target for the effect of LMB and more clinically relevant
XPO1 inhibitors, which covalently bind to this residue and block NES binding by physically occupying the
[45]
groove. In fact, experimental mutation of C528 renders cells resistant to these inhibitors .
Role of XPO1 in RNA nuclear export
Following transcription in the nucleus, active export to the cytoplasm is an essential step during the bio-
genesis of many classes of RNA, and/or a critical requirement for their function (recently reviewed by
[46]
Williams et al. ). Thus, messenger RNAs (mRNAs) need to be exported to undergo translation into pro-
teins, while ribosomal RNAs (rRNAs), transfer RNAs (tRNAs), small nuclear RNAs (snRNAs), microRNAs
(miRNAs) and long non-coding RNAs (lncRNAs) need to be transported to the cytoplasm in order to be
processed or to carry out their cellular activities. Nuclear export of RNA is a tightly regulated process that
involves the coordinated function of many different factors, including a large array of RNA-binding adap-
[6]
tor proteins as well as dedicated export receptors . XPO1 plays a pervasive role in this process, mediating
the nuclear export of several different classes of RNA.
XPO1 plays a prominent role in the export of 40S and 60S ribosomal subunits, containing rRNA, to the cy-
toplasm, which is a necessary step for their final maturation. The NES-containing protein Nmd3 functions
as the adaptor for 60S subunit export, while the adaptor involved in the export of the 40S subunit remains
to be identified .
[47]
In contrast to rRNAs, the vast majority of cellular mRNAs are exported to the cytoplasm by a receptor
[48]
unrelated to karyopherins, called NXF1, but XPO1 mediates nuclear export of a subset of mRNAs . Since