Page 96 - Read Online
P. 96
Author Instructions
the case of children, their parents or legal guardians. If the person has died, consent for publication must be obtained from
the next of kin of the participant. Manuscripts must include a statement that a written informed consent for publication was
obtained. Authors do not have to submit such content accompanying the manuscript. However, these documents must be
available if requested. If the manuscript does not involve this issue, state “Not applicable.” in this section.
2.3.3.8 Copyright
Authors retain copyright of their works through a Creative Commons Attribution 4.0 International License that clearly
states how readers can copy, distribute, and use their attributed research, free of charge. A declaration “© The Author(s)
2024.” will be added to each article. Authors are required to sign License to Publish before formal publication.
2.3.3.9 References
References should be numbered in order of appearance at the end of manuscripts. In the text, reference numbers should be
placed in square brackets and the corresponding references are cited thereafter. If the number of authors is less than or equal
to six, we require to list all authors’ names. If the number of authors is more than six, only the first three authors’ names are
required to be listed in the references, other authors’ names should be omitted and replaced with “et al.”. Abbreviations of
the journals should be provided on the basis of Index Medicus. Information from manuscripts accepted but not published
should be cited in the text as “Unpublished material” with written permission from the source.
References should be described as follows, depending on the types of works:
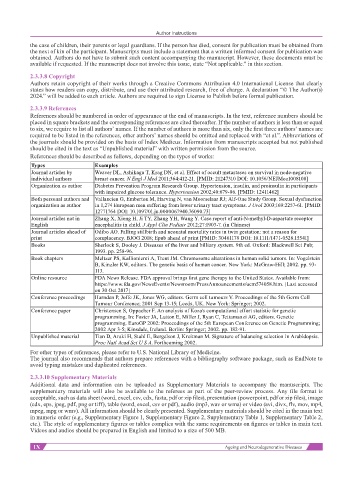
Types Examples
Journal articles by Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative
individual authors breast cancer. N Engl J Med 2011;364:412-21. [PMID: 21247310 DOI: 10.1056/NEJMoa1008108]
Organization as author Diabetes Prevention Program Research Group. Hypertension, insulin, and proinsulin in participants
with impaired glucose tolerance. Hypertension 2002;40:679-86. [PMID: 12411462]
Both personal authors and Vallancien G, Emberton M, Harving N, van Moorselaar RJ; Alf-One Study Group. Sexual dysfunction
organization as author in 1,274 European men suffering from lower urinary tract symptoms. J Urol 2003;169:2257-61. [PMID:
12771764 DOI: 10.1097/01.ju.0000067940.76090.73]
Journal articles not in Zhang X, Xiong H, Ji TY, Zhang YH, Wang Y. Case report of anti-N-methyl-D-aspartate receptor
English encephalitis in child. J Appl Clin Pediatr 2012;27:1903-7. (in Chinese)
Journal articles ahead of Odibo AO. Falling stillbirth and neonatal mortality rates in twin gestation: not a reason for
print complacency. BJOG 2018; Epub ahead of print [PMID: 30461178 DOI: 10.1111/1471-0528.15541]
Books Sherlock S, Dooley J. Diseases of the liver and billiary system. 9th ed. Oxford: Blackwell Sci Pub;
1993. pp. 258-96.
Book chapters Meltzer PS, Kallioniemi A, Trent JM. Chromosome alterations in human solid tumors. In: Vogelstein
B, Kinzler KW, editors. The genetic basis of human cancer. New York: McGraw-Hill; 2002. pp. 93-
113.
Online resource FDA News Release. FDA approval brings first gene therapy to the United States. Available from:
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm. [Last accessed
on 30 Oct 2017]
Conference proceedings Harnden P, Joffe JK, Jones WG, editors. Germ cell tumours V. Proceedings of the 5th Germ Cell
Tumour Conference; 2001 Sep 13-15; Leeds, UK. New York: Springer; 2002.
Conference paper Christensen S, Oppacher F. An analysis of Koza's computational effort statistic for genetic
programming. In: Foster JA, Lutton E, Miller J, Ryan C, Tettamanzi AG, editors. Genetic
programming. EuroGP 2002: Proceedings of the 5th European Conference on Genetic Programming;
2002 Apr 3-5; Kinsdale, Ireland. Berlin: Springer; 2002. pp. 182-91.
Unpublished material Tian D, Araki H, Stahl E, Bergelson J, Kreitman M. Signature of balancing selection in Arabidopsis.
Proc Natl Acad Sci U S A. Forthcoming 2002.
For other types of references, please refer to U.S. National Library of Medicine.
The journal also recommends that authors prepare references with a bibliography software package, such as EndNote to
avoid typing mistakes and duplicated references.
2.3.3.10 Supplementary Materials
Additional data and information can be uploaded as Supplementary Materials to accompany the manuscripts. The
supplementary materials will also be available to the referees as part of the peer-review process. Any file format is
acceptable, such as data sheet (word, excel, csv, cdx, fasta, pdf or zip files), presentation (powerpoint, pdf or zip files), image
(cdx, eps, jpeg, pdf, png or tiff), table (word, excel, csv or pdf), audio (mp3, wav or wma) or video (avi, divx, flv, mov, mp4,
mpeg, mpg or wmv). All information should be clearly presented. Supplementary materials should be cited in the main text
in numeric order (e.g., Supplementary Figure 1, Supplementary Figure 2, Supplementary Table 1, Supplementary Table 2,
etc.). The style of supplementary figures or tables complies with the same requirements on figures or tables in main text.
Videos and audios should be prepared in English and limited to a size of 500 MB.
IX Ageing and Neurodegenerative Diseases