Page 86 - Read Online
P. 86
Page 8 of 13 Zhang et al. Ageing Neur Dis 2023;3:24 https://dx.doi.org/10.20517/and.2023.18
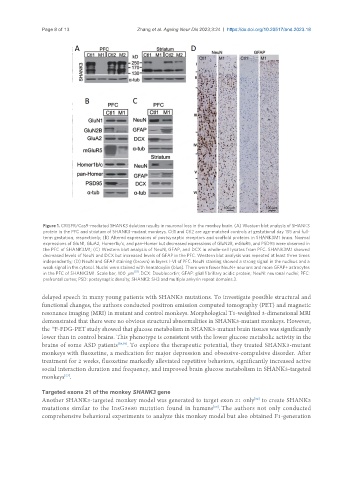
Figure 1. CRISPR/Cas9-mediated SHANK3 deletion results in neuronal loss in the monkey brain. (A) Western blot analysis of SHANK3
protein in the PFC and striatum of SHANK3-mutant monkeys. Ctl1 and Ctl2 are age-matched controls at gestational day 135 and full-
term gestation, respectively; (B) Altered expressions of postsynaptic receptors and scaffold proteins in SHANK3M1 brain. Normal
expressions of GluN1, GluA2, Homer1b/c, and pan-Homer but decreased expressions of GluN2B, mGluR5, and PSD95 were observed in
the PFC of SHANK3M1; (C) Western blot analysis of NeuN, GFAP, and DCX in whole-cell lysates from PFC. SHANK3M1 showed
decreased levels of NeuN and DCX but increased levels of GFAP in the PFC. Western blot analysis was repeated at least three times
independently; (D) NeuN and GFAP staining (brown) in layers I-VI of PFC. NeuN staining showed a strong signal in the nucleus and a
weak signal in the cytosol. Nuclei were stained with hematoxylin (blue). There were fewer NeuN+ neurons and more GFAP+ astrocytes
in the PFC of SHANK3M1. Scale bar, 100 μm [51] . DCX: Doublecortin; GFAP: glial fibrillary acidic protein; NeuN: neuronal nuclei; PFC:
prefrontal cortex; PSD: postsynaptic density; SHANK3: SH3 and multiple ankyrin repeat domains 3.
delayed speech in many young patients with SHANK3 mutations. To investigate possible structural and
functional changes, the authors conducted positron emission computed tomography (PET) and magnetic
resonance imaging (MRI) in mutant and control monkeys. Morphological T1-weighted 3-dimensional MRI
demonstrated that there were no obvious structural abnormalities in SHANK3-mutant monkeys. However,
the F-FDG-PET study showed that glucose metabolism in SHANK3-mutant brain tissues was significantly
18
lower than in control brains. This phenotype is consistent with the lower glucose metabolic activity in the
brains of some ASD patients [58,59] . To explore the therapeutic potential, they treated SHANK3-mutant
monkeys with fluoxetine, a medication for major depression and obsessive-compulsive disorder. After
treatment for 2 weeks, fluoxetine markedly alleviated repetitive behaviors, significantly increased active
social interaction duration and frequency, and improved brain glucose metabolism in SHANK3-targeted
monkeys .
[57]
Targeted exons 21 of the monkey SHANK3 gene
Another SHANK3-targeted monkey model was generated to target exon 21 only to create SHANK3
[52]
mutations similar to the InsG3680 mutation found in humans . The authors not only conducted
[60]
comprehensive behavioral experiments to analyze this monkey model but also obtained F1-generation