Page 61 - Read Online
P. 61
Sadagopan et al. Art Int Surg 2024;4:387-400 https://dx.doi.org/10.20517/ais.2024.34 Page 391
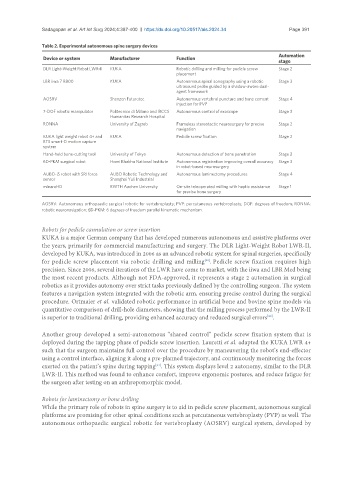
Table 2. Experimental autonomous spine surgery devices
Automation
Device or system Manufacturer Function
stage
DLR Light-Weight Robot LWR-II KUKA Robotic drilling and milling for pedicle screw Stage 2
placement
LBR iiwa 7 R800 KUKA Autonomous spinal sonography using a robotic Stage 3
ultrasound probe guided by a shadow-aware dual-
agent framework
AOSRV Shenzen Futuretec Autonomous vertebral puncture and bone cement Stage 4
injection for PVP
7-DOF robotic manipulator Politecnico di Milano and IRCCS Autonomous control of exoscope Stage 3
Humanitas Research Hospital
RONNA University of Zagreb Frameless stereotactic neurosurgery for precise Stage 2
navigation
KUKA light weight robot 4+ and KUKA Pedicle screw fixation Stage 2
BTS smart-D motion capture
system
Hand-held bone-cutting tool University of Tokyo Autonomous detection of bone penetration Stage 2
6D-PKM surgical robot Homi Bhabha National Institute Autonomous registration improving overall accuracy Stage 3
in robot-based neurosurgery
AUBO-i5 robot with SRI force AUBO Robotic Technology and Autonomous laminectomy procedures Stage 4
sensor Shanghai Yuli Industrial
minaroHD RWTH Aachen University On-site teleoperated milling with haptic assistance Stage 1
for precise bone surgery
AOSRV: Autonomous orthopaedic surgical robotic for vertebroplasty; PVP: percutaneous vertebroplasty; DOF: degrees of freedom; RONNA:
robotic neuronavigation; 6D-PKM: 6 degrees of freedom parallel kinematic mechanism.
Robots for pedicle cannulation or screw insertion
KUKA is a major German company that has developed numerous autonomous and assistive platforms over
the years, primarily for commercial manufacturing and surgery. The DLR Light-Weight Robot LWR-II,
developed by KUKA, was introduced in 2006 as an advanced robotic system for spinal surgeries, specifically
[20]
for pedicle screw placement via robotic drilling and milling . Pedicle screw fixation requires high
precision. Since 2006, several iterations of the LWR have come to market, with the iiwa and LBR Med being
the most recent products. Although not FDA-approved, it represents a stage 2 automation in surgical
robotics as it provides autonomy over strict tasks previously defined by the controlling surgeon. The system
features a navigation system integrated with the robotic arm, ensuring precise control during the surgical
procedure. Ortmaier et al. validated robotic performance in artificial bone and bovine spine models via
quantitative comparison of drill-hole diameters, showing that the milling process performed by the LWR-II
is superior to traditional drilling, providing enhanced accuracy and reduced surgical errors .
[20]
Another group developed a semi-autonomous “shared control” pedicle screw fixation system that is
deployed during the tapping phase of pedicle screw insertion. Lauretti et al. adapted the KUKA LWR 4+
such that the surgeon maintains full control over the procedure by maneuvering the robot’s end-effector
using a control interface, aligning it along a pre-planned trajectory, and continuously monitoring the forces
exerted on the patient’s spine during tapping . This system displays level 2 autonomy, similar to the DLR
[21]
LWR-II. This method was found to enhance comfort, improve ergonomic postures, and reduce fatigue for
the surgeon after testing on an anthropomorphic model.
Robots for laminectomy or bone drilling
While the primary role of robots in spine surgery is to aid in pedicle screw placement, autonomous surgical
platforms are promising for other spinal conditions such as percutaneous vertebroplasty (PVP) as well. The
autonomous orthopaedic surgical robotic for vertebroplasty (AOSRV) surgical system, developed by