Page 47 - Read Online
P. 47
Page 10 of 16 Fetro. Rare Dis Orphan Drugs J 2023;2:7 https://dx.doi.org/10.20517/rdodj.2023.06
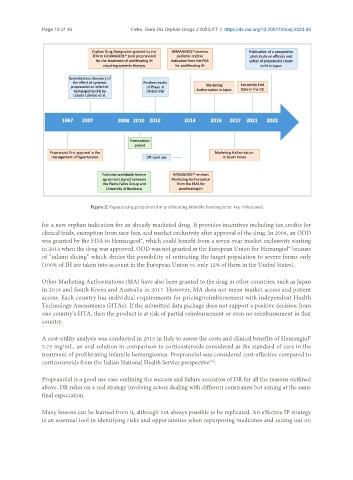
Figure 2. Repurposing propranolol in proliferating infantile hemangioma: key milestones.
for a new orphan indication for an already marketed drug. It provides incentives including tax credits for
clinical trials, exemption from user fees, and market exclusivity after approval of the drug. In 2008, an ODD
was granted by the FDA to Hemangeol®, which could benefit from a seven-year market exclusivity starting
in 2014 when the drug was approved. ODD was not granted in the European Union for Hemangiol® because
of “salami slicing” which denies the possibility of restricting the target population to severe forms only
(100% of IH are taken into account in the European Union vs. only 12% of them in the United States).
Other Marketing Authorizations (MA) have also been granted to the drug in other countries, such as Japan
in 2016 and South Korea and Australia in 2017. However, MA does not mean market access and patient
access. Each country has individual requirements for pricing/reimbursement with independent Health
Technology Assessments (HTAs). If the submitted data package does not support a positive decision from
one country’s HTA, then the product is at risk of partial reimbursement or even no reimbursement in that
country.
A cost-utility analysis was conducted in 2015 in Italy to assess the costs and clinical benefits of Hemangiol®
3.75 mg/mL, an oral solution in comparison to corticosteroids considered as the standard of care in the
treatment of proliferating infantile hemangiomas. Propranolol was considered cost-effective compared to
corticosteroids from the Italian National Health Service perspective .
[36]
Propranolol is a good use case outlining the success and failure scenarios of DR for all the reasons outlined
above. DR relies on a real strategy involving actors dealing with different constraints but aiming at the same
final expectation.
Many lessons can be learned from it, although not always possible to be replicated. An effective IP strategy
is an essential tool in identifying risks and opportunities when repurposing medicines and setting out on