Page 416 - Read Online
P. 416
Shamliyan et al. Vessel Plus 2020;4:35 I http://dx.doi.org/10.20517/2574-1209.2020.34 Page 7 of 13
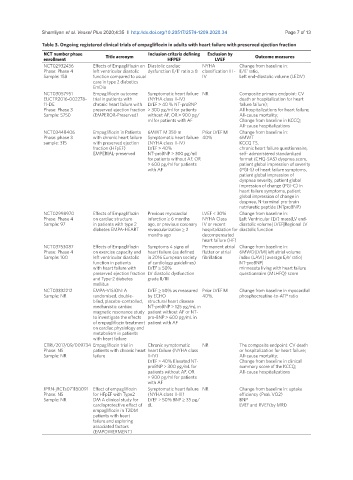
Table 3. Ongoing registered clinical trials of empagliflozin in adults with heart failure with preserved ejection fraction
NCT number phase Title acronym Inclusion criteria defining Exclusion by Outcome measures
enrollment HFPEF LVEF
NCT02932436 Effects of Empagliflozin on Diastolic cardiac NYHA Change from baseline in:
Phase: Phase 4 left ventricular diastolic dysfunction E/E’ ratio ≥ 8 classification III - E/E’ ratio,
Sample: 158 function compared to usual IV Left end-diastolic volume (LEDV)
care in type 2 diabetics
EmDia
NCT03057951 Empagliflozin outcome Symptomatic heart failure NR Composite primary endpoint: CV
EUCTR2016-002278- trial in patients with (NYHA class II-IV) death or hospitalization for heart
11-DE chronic heart failure with LVEF > 40 % NT-proBNP failure failure);
Phase: Phase 3 preserved ejection fraction > 300 pg/ml for patients All hospitalizations for heart failure;
Sample: 5750 (EMPEROR-Preserved) without AF, OR > 900 pg/ All-cause mortality;
ml for patients with AF Change from baseline in KCCQ;
All-cause hospitalizations
NCT03448406 Empagliflozin in Patients 6MWT ≤ 350 m Prior LVEF ≤ Change from baseline in:
Phase: phase 3 with chronic heart failure Symptomatic heart failure 40% 6MWT
sample: 315 with preserved ejection (NYHA class II-IV) KCCQ TS,
fraction (HFpEF) LVEF > 40% chronic heart failure questionnaire,
EMPERIAL-preserved NT-proBNP > 300 pg/ml self- administered standardized
for patients without AF, OR format (CHQ-SAS) dyspnea score,
> 600 pg/ml for patients patient global impression of severity
with AF (PGI-S) of heart failure symptoms,
patient global impression of
dyspnea severity, patient global
impression of change (PGI-C) in
heart failure symptoms, patient
global impression of change in
dyspnea, N-terminal pro-brain
natriuretic peptide (NTproBNP)
NCT02998970 Effects of Empagliflozin Previous myocardial LVEF < 30% Change from baseline in:
Phase: Phase 4 on cardiac structure infarction ≥ 6 months NYHA Class Left Ventricular (LV) mass|LV end-
Sample: 97 in patients with type 2 ago, or previous coronary IV or recent diastolic volume| LVEF|Regional LV
diabetes EMPA-HEART revascularization ≥ 2 hospitalization for diastolic function
months ago decompensated
heart failure (HF)
NCT03753087 Effects of Empagliflozin Symptoms ± signs of Permanent atrial Change from baseline in:
Phase: Phase 4 on exercise capacity and heart failure (as defined flutter or atrial 6MWD|LVMI|left atrial volume
Sample: 100 left ventricular diastolic in 2016 European society fibrillation index (LAVI)| average E/e’ ratio|
function in patients of cardiology guidelines) NT-proBNP|
with heart failure with LVEF ≥ 50% minnesota living with heart failure
preserved ejection fraction LV diastolic dysfunction questionnaire (MLHFQ) score
and Type-2 diabetes grade II/III
mellitus
NCT03332212 EMPA-VISION: A LVEF ≥ 50% as measured Prior LVEF ≤ Change from baseline in myocardial
Sample: NR randomised, double- by ECHO 40%. phosphocreatine-to-ATP ratio
blind, placebo-controlled, structural heart disease
mechanistic cardiac NT-proBNP > 125 pg/mL in
magnetic resonance study patient without AF or NT-
to investigate the effects pro-BNP > 600 pg/mL in
of empagliflozin treatment patient with AF
on cardiac physiology and
metabolism in patients
with heart failure
CTRI/2017/09/009734 Empagliflozin trial in Chronic symptomatic NR The composite endpoint: CV death
Phase: NS patients with chronic heart heart failure (NYHA class or hospitalization for heart failure;
Sample: NR failure II-IV) All-cause mortality;
LVEF > 40% Elevated NT- Change from baseline in clinical
proBNP > 300 pg/mL for summary score of the KCCQ;
patients without AF, OR All-cause hospitalizations
> 900 pg/ml for patients
with AF
JPRN-jRCTs071180091 Effect of empagliflozin Symptomatic heart failure NR Change from baseline in: uptake
Phase: NS for HFpEF with Type2 (NYHA class II-III) efficiency (Peak VO2)
Sample: NR DM A clinical study for LVEF > 50% BNP ≥ 35 pg/ BNP
cardioprotective effect of dL LVEF and RVEF(by MRI)
empagliflozin in T2DM
patients with heart
failure and exploring
associated factors
(EMPOWERMENT)