Page 415 - Read Online
P. 415
Page 6 of 13 Shamliyan et al. Vessel Plus 2020;4:35 I http://dx.doi.org/10.20517/2574-1209.2020.34
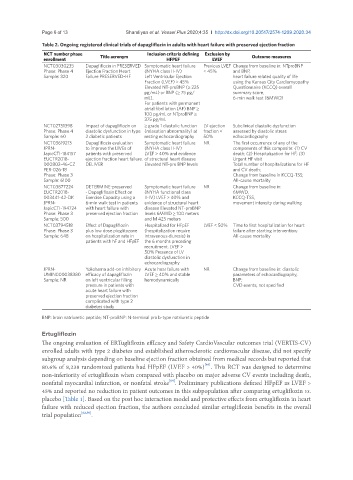
Table 2. Ongoing registered clinical trials of dapagliflozin in adults with heart failure with preserved ejection fraction
NCT number phase Title acronym Inclusion criteria defining Exclusion by Outcome measures
enrollment HFPEF LVEF
NCT03030235 Dapagliflozin in PRESERVED Symptomatic heart failure Previous LVEF Change from baseline in: NTproBNP
Phase: Phase 4 Ejection Fraction Heart (NYHA class II-IV) < 45% and BNP,
Sample: 320 Failure PRESERVED-HF Left Ventricular Ejection heart failure related quality of life
Fraction (LVEF) ≥ 45% using the Kansas City Cardiomyopathy
Elevated NT-proBNP (≥ 225 Questionnaire (KCCQ) overall
pg/mL) or BNP (≥ 75 pg/ summary score,
mL). 6-min walk test (6MWD)
For patients with permanent
atrial fibrillation (AF) BNP ≥
100 pg/mL or NTproBNP ≥
375 pg/mL
NCT02751398 Impact of dapagliflozin on ≥ grade 1 diastolic function LV ejection Subclinical diastolic dysfunction
Phase: Phase 4 diastolic dysfunction in type (relaxation abnormality) at fraction < assessed by diastolic stress
Sample: 60 2 diabetic patients resting echocardiography 50% echocardiography
NCT03619213 Dapagliflozin evaluation Symptomatic heart failure NR The first occurrence of any of the
JPRN- to improve the LIVEs of (NYHA class II-IV) components of this composite: (1) CV
JapicCTI-184157 patients with preserved LVEF > 40% and evidence death; (2) Hospitalization for HF; (3)
EUCTR2018- ejection fraction heart failure. of structural heart disease Urgent HF visit
000802-46-CZ DELIVER Elevated NT-pro BNP levels Total number of hospitalizations for HF
PER-026-18 and CV death;
Phase: Phase 3 Change from baseline in KCCQ-TSS;
Sample: 6100 All-cause mortality
NCT03877224 DETERMINE-preserved Symptomatic heart failure NR Change from baseline in:
EUCTR2018- - Dapagliflozin Effect on (NYHA functional class 6MWD,
003441-42-DK Exercise Capacity using a II-IV) LVEF > 40% and KCCQ-TSS,
JPRN- 6-min walk test in patients evidence of structural heart movement intensity during walking
JapicCTI-194724 with heart failure with disease Elevated NT-proBNP
Phase: Phase 3 preserved ejection fraction levels 6MWD ≥ 100 meters
Sample: 500 and ≤ 425 meters
NCT03794518 Effect of Dapagliflozin Hospitalized for HFpEF LVEF < 50% Time to first hospitalization for heart
Phase: Phase 3 plus low dose pioglitazone (hospitalization require failure after starting intervention;
Sample: 648 on hospitalization rate in intravenous diuresis) in All-cause mortality
patients with hF and HFpEF the 6 months preceding
recruitment. LVEF >
50% Presence of LV
diastolic dysfunction in
echocardiography
JPRN- Yokohama add-on inhibitory Acute hear failure with NR Change from baseline in: diastolic
UMIN000038380 efficacy of dapagliflozin LVEF ≥ 40% and stable parameters of echocardiography,
Sample: NR on left ventricular filling hemodynamically BNP;
pressure in patients with CVD events, not specified
acute heart failure with
preserved ejection fraction
complicated with type 2
diabetes study
BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro b-type natriuretic peptide
Ertugliflozin
The ongoing evaluation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV)
enrolled adults with type 2 diabetes and established atherosclerotic cardiovascular disease, did not specify
subgroup analysis depending on baseline ejection fraction obtained from medical records but reported that
[69]
80.6% of 8,238 randomized patients had HFpEF (LVEF > 40%) . This RCT was designed to determine
non-inferiority of ertugliflozin when compared with placebo on major adverse CV events including death,
[69]
nonfatal myocardial infarction, or nonfatal stroke . Preliminary publications defined HFpEF as LVEF >
45% and reported no reduction in patient outcomes in this subpopulation after comparing ertugliflozin vs.
placebo [Table 1]. Based on the post hoc interaction model and protective effects from ertugliflozin in heart
failure with reduced ejection fraction, the authors concluded similar ertugliflozin benefits in the overall
trial population [62,70] .