Page 48 - Read Online
P. 48
Page 4 of 7 D’Abramo et al. Vessel Plus 2019;3:4 I http://dx.doi.org/10.20517/2574-1209.2018.41
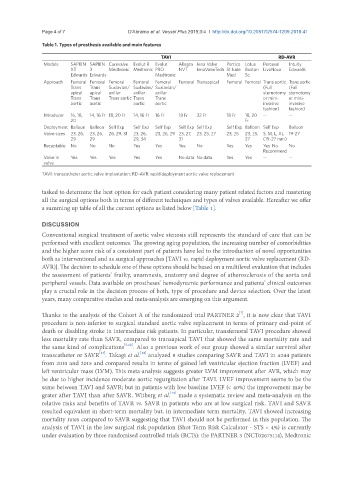
Table 1. Types of prosthesis available and main features
TAVI RD-AVR
Models SAPIEN SAPIEN Corevalve Evolut R Evolut Allegra Jena Valve Portico Lotus Perceval Intuity
XT 3 Medtronic Medtronic PRO NVT JenaValveTech St Jude Boston LivaNova Edwards
Edwards Edwards Medtronic Med Sc.
Approach Femoral Femoral Femoral Femoral Femoral Femoral Transapical Femoral Femoral Trans aortic Trans aortic
Trans Trans Suxlavian/ Suxlavian/ Suxlavian/ (Full (Full
apical apical axillar axillar axillar sternotomy sternotomy
Trans Trans Trans aortic Trans Trans or mini- or mini-
aortic aortic aortic aortic invasive invasive
fashion) fashion)
Introducer 16, 18, 14, 16 Fr 18, 20 Fr 14, 16 Fr 16 Fr 18 Fr 32 Fr 18 Fr 18, 20 -- --
20 Fr
Deployment Balloon Balloon Self Exp Self Exp Self Exp Self Exp Self Exp Self Exp Balloon Self Exp Balloon
Valve sizes 23, 26, 23, 26, 26, 29, 31 23, 26, 23, 26, 29 23, 27, 23, 25, 27 23, 25 23, 25, S, M, L, XL 19-27
29 29 29, 34 31 27 (19-27 mm)
Recaptable No No No Yes Yes Yes No Yes Yes Yes No No
Recommend
Valve in Yes Yes Yes Yes Yes No data No data Yes Yes -- --
valve
TAVI: transcatheter aortic valve implantation; RD-AVR: rapid deployment aortic valve replacement
tasked to determine the best option for each patient considering many patient related factors and mastering
all the surgical options both in terms of different techniques and types of valves available. Hereafter we offer
a summing up table of all the current options as listed below [Table 1].
DISCUSSION
Conventional surgical treatment of aortic valve stenosis still represents the standard of care that can be
performed with excellent outcomes. The growing aging population, the increasing number of comorbidities
and the higher score risk of a consistent part of patients have led to the introduction of novel opportunities
both as interventional and as surgical approaches [TAVI vs. rapid deployment aortic valve replacement (RD-
AVR)]. The decision to schedule one of these options should be based on a multilevel evaluation that includes
the assessment of patients’ frailty, anamnesis, anatomy and degree of atherosclerosis of the aorta and
peripheral vessels. Data available on prostheses’ hemodynamic performance and patients’ clinical outcomes
play a crucial role in the decision process of both, type of procedure and device selection. Over the latest
years, many comparative studies and meta-analysis are emerging on this argument.
[7]
Thanks to the analysis of the Cohort A of the randomized trial PARTNER 2 , it is now clear that TAVI
procedure is non-inferior to surgical standard aortic valve replacement in terms of primary end-point of
death or disabling stroke in intermediate risk patients. In particular, transfemoral TAVI procedure showed
less mortality rate than SAVR, compared to transapical TAVI that showed the same mortality rate and
the same kind of complications [7,12] . Also a previous work of our group showed a similar survival after
[14]
[13]
transcatheter or SAVR . Takagi et al. analyzed 8 studies comparing SAVR and TAVI in 4244 patients
from 2010 and 2015 and compared results in terms of gained left ventricular ejection fraction (LVEF) and
left ventricular mass (LVM). This meta-analysis suggests greater LVM improvement after AVR, which may
be due to higher incidence moderate aortic regurgitation after TAVI. LVEF improvement seems to be the
same between TAVI and SAVR; but in patients with low baseline LVEF (< 40%) the improvement may be
[15]
grater after TAVI than after SAVR. Witberg et al. made a systematic review and meta-analysis on the
relative risks and benefits of TAVR vs. SAVR in patients who are at low surgical risk. TAVI and SAVR
resulted equivalent in short-term mortality but, in intermediate term mortality, TAVI showed increasing
mortality rates compared to SAVR suggesting that TAVI should not be performed in this population. The
analysis of TAVI in the low surgical risk population (Shot Term Risk Calculator - STS < 4%) is currently
under evaluation by three randomised controlled trials (RCTs): the PARTNER 3 (NCT02675114), Medtronic