Page 333 - Read Online
P. 333
Page 4 of 8 Lancaster et al. Vessel Plus 2019;3:34 I http://dx.doi.org/10.20517/2574-1209.2019.16
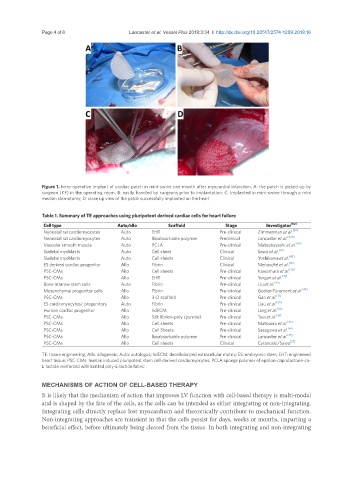
Figure 1. Intra-operative implant of cardiac patch in mini-swine one month after myocardial infarction. A: the patch is picked up by
surgeon (KF) in the operating room; B: easily handled by surgeons prior to implantation; C: implanted in mini-swine through a mini
median sternotomy; D: close up view of the patch successfully implanted on the heart
Table 1. Summary of TE approaches using pluripotent derived cardiac cells for heart failure
Cell type Auto/allo Scaffold Stage Investigator (Ref)
Neonatal rat cardiomyocytes Auto EHT Pre-clinical Zimmerman et al. [39]
Neonatal rat cardiomyocytes Auto Bioabsorbable polymer Preclinical Lancaster et al. [15,16]
Vascular smooth muscle Auto PCLA Pre-clinical Matsubayashi et al. [40]
Skeletal myoblasts Auto Cell sheet Clinical Sawa et al. [41]
Skeletal myoblasts Auto Cell sheets Clinical Yoshikawa et al. [42]
ES derived cardiac progenitor Allo Fibrin Clinical Menasché et al. [19]
PSC-CMs Allo Cell sheets Pre-clinical Kawamura et al. [20]
PSC-CMs Allo EHT Pre-clinical Yorgan et al. [43]
Bone marrow stem cells Auto Fibrin Pre-clinical Liu et al. [44]
Mesenchymal progenitor cells Allo Fibrin Pre-clinical Godier-Furemont et al. [45]
PSC-CMs Allo 3-D scaffold Pre-clinical Gao et al. [17]
ES cardiomyocytes/ progenitors Auto Fibrin Pre-clinical Liau et al. [29]
Human cardiac progenitor Allo hdECM Pre-clinical Jang et al. [32]
PSC-CMs Allo Silk fibrion-poly (pyrrole) Pre-clinical Tsui et al. [33]
PSC-CMs Allo Cell sheets Pre-clinical Matsuura et al. [46]
PSC-CMs Allo Cell Sheets Pre-clinical Sasagawa et al. [47]
PSC-CMs Allo Bioabsorbable polymer Pre-clinical Lancaster et al. [35]
PSC-CMs Allo Cell sheets Clinical Cyranoski/Sawa [27]
TE: tissue engineering; Allo: allogeneic; Auto: autologus; hdECM: decellularized extracellular matrix; ES: embryonic stem; EHT: engineered
heart tissue; PSC-CMs: human induced pluripotent stem cell-derived cardiomyocytes; PCLA:sponge polymer of epsilon-caprolactone-co-
L-lactide reinforced with knitted poly-L-lactide fabric
MECHANISMS OF ACTION OF CELL-BASED THERAPY
It is likely that the mechanism of action that improves LV function with cell-based therapy is multi-modal
and is shaped by the fate of the cells, as the cells can be intended as either integrating or non-integrating.
Integrating cells directly replace lost myocardium and theoretically contribute to mechanical function.
Non-integrating approaches are transient in that the cells persist for days, weeks or months, imparting a
beneficial effect, before ultimately being cleared from the tissue. In both integrating and non-integrating