Page 166 - Read Online
P. 166
Page 6 of 9 Rove et al. Vessel Plus 2022;6:55 https://dx.doi.org/10.20517/2574-1209.2021.133
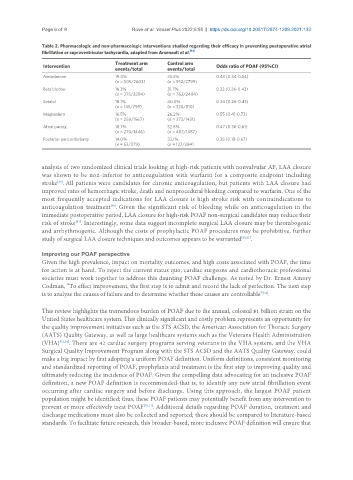
Table 2. Pharmacologic and non-pharmacologic interventions studied regarding their efficacy in preventing postoperative atrial
fibrillation or supraventricular tachycardia, adapted from Arsenault et al. [14]
Treatment arm Control arm
Intervention Odds ratio of POAF (95%CI)
events/total events/total
Amiodarone 19.4% 33.3% 0.43 (0.34-0.54)
(n = 505/2603) (n = 932/2799)
Beta blocker 16.3% 31.7% 0.33 (0.26-0.43)
(n = 375/2294) (n = 762/2404)
Sotalol 18.1% 40.0% 0.34 (0.26-0.43)
(n = 145/799) (n = 324/810)
Magnesium 16.5% 26.2% 0.55 (0.41-0.73)
(n = 258/1567) (n = 373/1421)
Atrial pacing 18.7% 32.8% 0.47 (0.36-0.61)
(n = 270/1446) (n = 487/1487)
Posterior pericardiotomy 14.0% 33.1% 0.35 (0.18-0.67)
(n = 53/379) (n = 127/384)
analysis of two randomized clinical trials looking at high-risk patients with nonvalvular AF, LAA closure
was shown to be non-inferior to anticoagulation with warfarin for a composite endpoint including
stroke . All patients were candidates for chronic anticoagulation, but patients with LAA closure had
[29]
improved rates of hemorrhagic stroke, death and nonprocedural bleeding compared to warfarin. One of the
most frequently accepted indications for LAA closure is high stroke risk with contraindications to
anticoagulation treatment . Given the significant risk of bleeding while on anticoagulation in the
[30]
immediate postoperative period, LAA closure for high-risk POAF non-surgical candidates may reduce their
risk of stroke . Interestingly, some data suggest incomplete surgical LAA closure may be thrombogenic
[31]
and arrhythmogenic. Although the costs of prophylactic POAF procedures may be prohibitive, further
study of surgical LAA closure techniques and outcomes appears to be warranted [30,31] .
Improving our POAF perspective
Given the high prevalence, impact on mortality outcomes, and high costs associated with POAF, the time
for action is at hand. To reject the current status quo, cardiac surgeons and cardiothoracic professional
societies must work together to address this daunting POAF challenge. As noted by Dr. Ernest Amory
Codman, “To effect improvement, the first step is to admit and record the lack of perfection. The next step
is to analyze the causes of failure and to determine whether these causes are controllable” .
[32]
This review highlights the tremendous burden of POAF due to the annual, colossal $1 billion strain on the
United States healthcare system. This clinically significant and costly problem represents an opportunity for
the quality improvement initiatives such as the STS ACSD, the American Association for Thoracic Surgery
(AATS) Quality Gateway, as well as large healthcare systems such as the Veterans Health Administration
(VHA) [33,34] . There are 42 cardiac surgery programs serving veterans in the VHA system, and the VHA
Surgical Quality Improvement Program along with the STS ACSD and the AATS Quality Gateway, could
make a big impact by first adopting a uniform POAF definition. Uniform definitions, consistent monitoring
and standardized reporting of POAF, prophylaxis and treatment is the first step to improving quality and
ultimately reducing the incidence of POAF. Given the compelling data advocating for an inclusive POAF
definition, a new POAF definition is recommended-that is, to identify any new atrial fibrillation event
occurring after cardiac surgery and before discharge. Using this approach, the largest POAF patient
population might be identified; thus, these POAF patients may potentially benefit from any intervention to
prevent or more effectively treat POAF [10,11] . Additional details regarding POAF duration, treatment and
discharge medications must also be collected and reported; these should be compared to literature-based
standards. To facilitate future research, this broader-based, more inclusive POAF definition will ensure that